Human umbilical cord mesenchymal stem cell-derived exosomes promote osteogenesis in glucocorticoid-induced osteoporosis through PI3K/AKT signaling pathway-mediated ferroptosis inhibition
- PMID: 40257841
- PMCID: PMC12010878
- DOI: 10.1093/stcltm/szae096
Human umbilical cord mesenchymal stem cell-derived exosomes promote osteogenesis in glucocorticoid-induced osteoporosis through PI3K/AKT signaling pathway-mediated ferroptosis inhibition
Abstract
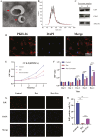
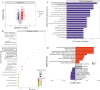
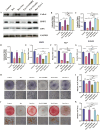
Glucocorticoid-induced osteoporosis (GIOP), the most common cause of secondary osteoporosis, is characterized by significant bone loss, decreased bone quality, and increased fracture risk. The current treatments for GIOP have several drawbacks. Exosomes are vital for cellular processes. However, very few studies have focused on using human umbilical cord mesenchymal stem cell-derived exosomes (hUCMSC-EXOs) for GIOP treatment. In vitro and in vivo dexamethasone was used to evaluate the therapeutic effects of hUCMSC-EXOs on GIOP. CCK-8 and EdU assays were used to evaluate cell viability and proliferation, respectively. We conducted an alkaline phosphatase activity assay, alizarin red staining, Western blotting, and real-time PCR to detect the effect on osteogenesis. TMT-labeled quantitative proteomic and bioinformatic analyses were performed. Furthermore, we performed Western blotting, immunofluorescence, reactive oxygen species assays, and lipid peroxidation assays to investigate the regulatory mechanism by which hUCMSC-EXOs affect cell proliferation and osteogenic differentiation. The in vivo effects of hUCMSC-EXOs were evaluated using micro-CT, hematoxylin, and eosin staining, and immunohistochemical staining. We found that hUCMSC-EXOs reversed the inhibitory effects of glucocorticoids on human bone marrow stromal cell (hBMSC) proliferation and osteogenic differentiation and demonstrated that hUCMSC-EXOs reversed GIOP via the PI3K/AKT signaling pathway, inhibiting lipid peroxidation in vitro and in vivo. HUCMSC-EXOs promote hBMSC osteogenesis through the PI3K/AKT signaling pathway, inhibit ferroptosis, and have therapeutic potential for GIOP in mice.
Keywords: PI3K/AKT signaling; exosome; ferroptosis; human bone marrow stromal cells; human umbilical cord mesenchymal stem cells; osteogenesis.
Published by Oxford University Press 2025.
Conflict of interest statement
The authors declared no potential conflicts of interest.
Figures





Similar articles
-
Exosomes derived from umbilical cord mesenchymal stem cells alleviate jaw bone marrow mesenchymal stem cells senescence and restore osteogenic differentiation potential.Stem Cell Res Ther. 2025 Aug 29;16(1):475. doi: 10.1186/s13287-025-04587-w. Stem Cell Res Ther. 2025. PMID: 40877889 Free PMC article.
-
Therapeutic Effects of Mechanical Stress-Induced C2C12-Derived Exosomes on Glucocorticoid-Induced Osteoporosis Through miR-92a-3p/PTEN/AKT Signaling Pathway.Int J Nanomedicine. 2023 Dec 12;18:7583-7603. doi: 10.2147/IJN.S435301. eCollection 2023. Int J Nanomedicine. 2023. PMID: 38106447 Free PMC article.
-
Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Enhance the Osteoblastic Differentiation of Periodontal Ligament Stem Cells Under High Glucose Conditions Through the PI3K/AKT Signaling Pathway.Biomed Environ Sci. 2022 Sep 20;35(9):811-820. doi: 10.3967/bes2022.105. Biomed Environ Sci. 2022. PMID: 36189996
-
Research landscape and trends of human umbilical cord mesenchymal stem cell-derived exosomes.Stem Cell Res Ther. 2025 May 28;16(1):259. doi: 10.1186/s13287-025-04379-2. Stem Cell Res Ther. 2025. PMID: 40437553 Free PMC article. Review.
-
The Role of Ferroptosis in Osteoporosis and Advances in Chinese Herbal Interventions.Biology (Basel). 2025 Apr 2;14(4):367. doi: 10.3390/biology14040367. Biology (Basel). 2025. PMID: 40282232 Free PMC article. Review.
References
-
- Adami G, Saag KG.. Glucocorticoid-induced osteoporosis update. Curr Opin Rheumatol. 2019;31:388-393. https://doi.org/10.1097/BOR.0000000000000608 - DOI - PubMed
-
- Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C.. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993-1000. https://doi.org/10.1359/jbmr.2000.15.6.993 - DOI - PubMed
-
- Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62-70. https://doi.org/10.1056/NEJMcp1012926 - DOI - PubMed
-
- Buckley L, Humphrey MB.. Glucocorticoid-induced osteoporosis. N Engl J Med. 2018;379:2547-2556. https://doi.org/10.1056/NEJMcp1800214 - DOI - PubMed
-
- Mudano A, Allison J, Hill J, Rothermel T, Saag K.. Variations in glucocorticoid induced osteoporosis prevention in a managed care cohort. J Rheumatol. 2001;28:1298-1305. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

