TGF-β expressed by M2 macrophages promotes wound healing by inhibiting TSG-6 expression by mesenchymal stem cells
- PMID: 40257993
- PMCID: PMC12011265
- DOI: 10.1371/journal.pone.0316692
TGF-β expressed by M2 macrophages promotes wound healing by inhibiting TSG-6 expression by mesenchymal stem cells
Abstract
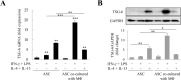
Wound healing involves the collaboration of multiple cells, including macrophages and fibroblasts, and requires the coordination of cytokines, growth factors, and matrix proteins to regulate the repair response. In this study, we investigated how M2 macrophages regulate expression of the anti-fibrotic and anti-inflammatory regulator tumor necrosis factor-α (TNF-α)-stimulated gene 6 (TSG-6) secreted by adipose tissue-derived stem cells (ASCs) during wound healing. Interleukin (IL)-4/IL-13, which is used to differentiate macrophage M2 phenotypes, increases TSG-6 in ASCs; however, M2 macrophages significantly decrease TSG-6 in ASCs. Transforming growth factor (TGF)-β expression was increased, and TNF-α expression was decreased in M2 macrophages. TGF-β inhibited IL-4/IL-13-induced ASC TSG-6 expression. In addition, TSG-6 suppressed TGF-β-triggered wound closure and fibrogenic responses in LX-2 cells. Collectively, TSG-6 inhibited wound healing, but M2 macrophage-expressed TGF-β prevented TSG-6 production from ASCs, which ultimately helped wound healing. Our results indicate that the balance of TNF-α and TGF-β levels during wound healing regulates TSG-6 production from ASCs, which may ultimately modulate the healing process. Our study findings could contribute to novel therapeutic strategies that manipulate the delicate balance between TNF-α and TGF-β to enhance wound repair and mitigate fibrosis.
Copyright: © 2025 Eom et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2005;31(6):674–86; discussion 86. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

