CRMP2 phosphorylation regulates polarization and spinal infiltration of CD4 + T lymphocytes, inhibits spinal glial activation, and arthritic pain
- PMID: 40258133
- PMCID: PMC12363488
- DOI: 10.1097/j.pain.0000000000003599
CRMP2 phosphorylation regulates polarization and spinal infiltration of CD4 + T lymphocytes, inhibits spinal glial activation, and arthritic pain
Abstract
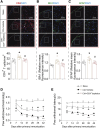
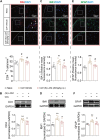
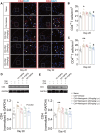
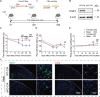
Chronic pain, a hallmark symptom of rheumatoid arthritis (RA), is strongly linked to central sensitization driven by spinal glial cell activation. Despite its clinical significance, the precise mechanisms remain unclear. Recent findings highlight the crucial role of interactions between circulating monocytes and central nervous system glial cells in chronic pain associated with autoimmune conditions. Our study focuses on CD4 + T-cell infiltration into the spinal dorsal horn (SDH) after collagen-induced arthritis (CIA) immunization. Immunohistochemistry results indicate that CD4 + T cells are critical in initiating arthritic pain. Intrathecal injection of CD4 + T cells in naïve mice induced glial activation and pain-like behaviors, while neutralizing antibodies suppressed these effects. Elevated phosphorylation of collapsin response mediator protein 2 (CRMP2) in CIA-derived CD4 + T lymphocytes was closely associated with pathological spinal infiltration. To modulate CRMP2 phosphorylation, we used naringenin (NAR), a known CRMP2 regulator, and (S)-Lacosamide ((S)-LCM), a specific inhibitor of phosphorylated CRMP2. Both compounds reduced CD4 + T-cell infiltration into the SDH and attenuated central sensitization in CIA rats. CRMP2 conditional knockout (cKO) in CD4 + T cells significantly alleviated arthritic pain. In addition, in vitro blood brain barrier models and Transwell assays showed impaired CD4 + T-cell migration and transendothelial invasion upon cKO or treatment with NAR and (S)-LCM. These interventions also decreased the proportion of polarized CD4 + T cells in CIA-induced mice. Our research highlights the role of CRMP2 phosphorylation in CD4 + T-cell behavior, spinal infiltration, and pain modulation, suggesting potential novel therapeutic strategies for RA-associated chronic pain.
Keywords: CRMP2; Central sensitization; Chronic pain; Rheumatoid arthritis.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association for the Study of Pain.
Conflict of interest statement
The authors declare that they have no known competing financial interests.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures



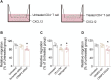
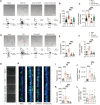

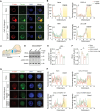
References
-
- Baig S, DiRenzo DD. Complementary and alternative medicine use in rheumatoid arthritis. Curr Rheumatol Rep 2020;22:61. - PubMed
MeSH terms
Substances
Grants and funding
- 2022YFC3501204/Grants of National Key R&D Program of China
- GZY-ZJ-KJ-23009/Science and Technology Co-constructed Plan
- LY22H280008/Natural Science Foundation of Zhejiang Province
- 82204649/National Natural Science Foundation of China
- LTGY24H280001/Natural Science Foundation of Zhejiang Province
- 2022JKZKTS04/Foundation of Zhejiang Chinese Medical University
- 2023JKZKTS38/Foundation of Zhejiang Chinese Medical University
- 82405298/National Natural Science Foundation of China
- LQ24H270006/Natural Science Foundation of Zhejiang Province
- 2022RCZXZK29/Foundation of Zhejiang Chinese Medical University
- 2024ZF063/Zhejiang Traditional Medicine and Technology Program
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

