Composite Confirmed Disability Worsening/Progression Is a Useful Clinical Endpoint for Multiple Sclerosis Clinical Trials
- PMID: 40258203
- PMCID: PMC12136489
- DOI: 10.1212/WNL.0000000000213558
Composite Confirmed Disability Worsening/Progression Is a Useful Clinical Endpoint for Multiple Sclerosis Clinical Trials
Abstract
Background and objectives: Sensitive and meaningful disability worsening measures remain an unmet medical need in multiple sclerosis (MS). Composite confirmed disability worsening/progression (cCDW/cCDP) combines the Expanded Disability Status Scale (EDSS) with performance tests of ambulation and dexterity (Timed 25-Foot Walk Test [T25FWT] and Nine-Hole Peg Test [9HPT]). We assessed the relation of changes in these measures to understand the utility of cCDW/cCDP as an endpoint for MS trials.
Methods: Clinical trials measuring all components of cCDW were selected for the analysis of (i) individual patient-level data from Roche-sponsored MS studies to characterize the association between performance-test changes and subsequent EDSS changes and (ii) population-level data from published studies reporting treatment effects on EDSS and either cCDP or T25FWT or 9HPT events to examine the relationship between treatment effects on T25FWT and EDSS events.
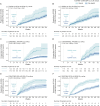
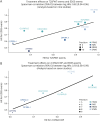
Results: Analysis (i): 6 Roche-sponsored Phase III trials comprising 4,979 patients with relapsing-remitting MS (RRMS; n = 1,225), relapsing MS (RMS; n = 1,656), progressive MS (PMS; n = 922), and primary progressive MS (PPMS; n = 1,171), with a data cutoff of November 2022, were included in the individual patient analyses. For all trials, T25FWT events were associated with increased risk of subsequent EDSS events (hazard ratios [HRs], p values: 2.11-5.20, 0.07-<0.001); similar associations were found for 9HPT events with HRs for later EDSS events ranging from 1.47 to 2.66 (p values from 0.24-<0.001). For patients without EDSS events in the first 96 study weeks, T25FWT or 9HPT events in the first 96 study weeks were associated with increased risk of subsequent EDSS events (HRs, p values: T25FWT 1.74-3.26, 0.01-<0.001; 9HPT 1.45-3.08, 0.45-<0.001). Patients with T25FWT or 9HPT events were more likely to experience a ≥8-point change from baseline at the final visit in the 29-item Multiple Sclerosis Impact Scale physical subscale (risk ratios, p values: T25FWT 1.45-2.17, 0.004-<0.001; 9HPT 1.26-1.87, 0.15-0.03). Analysis (ii): In the 9 studies included, treatment effects on T25FWT events were predictive of treatment effects on EDSS events (Spearman correlation [95% CI] = 0.82 [0.34-0.96], p = 0.005).
Discussion: In this post hoc analysis, worsening on T25FWT or 9HPT was a harbinger of EDSS worsening and treatment effects on T25FWT correlated with those on EDSS. These results establish the predictive validity and clinical relevance of performance-test worsening, thus supporting use of cCDW/cCDP as a primary outcome for progression in MS trials.
Clinical trial identifiers: ClinicalTrials.gov Identifiers: NCT01247324 (OPERA I); first submitted November 23, 2010; first patient enrolled: August 31, 2011; available at clinicaltrials.gov/study/NCT01247324. NCT01412333 (OPERA II); first submitted August 8, 2011; first patient enrolled: September 20, 2011; available at clinicaltrials.gov/study/NCT01412333. NCT03085810 (ENSEMBLE); first submitted March 16, 2017; first patient enrolled: March 27, 2017; available at clinicaltrials.gov/study/NCT03085810. NCT01194570 (ORATORIO); first submitted August 28, 2010; first patient enrolled: March 3, 2011; available at clinicaltrials.gov/study/NCT01194570. NCT03523858 (CONSONANCE); first submitted April 16, 2018; first patient enrolled: May 28, 2018; available at clinicaltrials.gov/study/NCT03523858. NCT00087529 (OLYMPUS); first submitted July 9, 2004; first patient enrolled: July 9, 2004; available at clinicaltrials.gov/study/NCT00087529.
Conflict of interest statement
L. Kappos has received no personal compensation. His institutions (University Hospital Basel/Foundation Clinical Neuroimmunology and Neuroscience Basel) have received and used exclusively for research support: payments for steering committee and advisory board participation, consultancy services, and participation in educational activities from Actelion, Bayer, Bristol Myers Squibb, df-mp Molnia & Pohlmann, Celgene, Eli Lilly, EMD Serono, Genentech Inc., GlaxoSmithKline, Janssen, Japan Tobacco, Merck, MH Consulting, Minoryx, Novartis, F. Hoffmann-La Roche Ltd, Senda Biosciences Inc., Sanofi, Santhera, Shionogi BV, TG Therapeutics, and Wellmera; license fees for Neurostatus-UHB products; and grants from Novartis, Innosuisse, and Roche. S. Yiu is an employee of Roche Products Ltd and a shareholder in F. Hoffmann-La Roche Ltd. F. Dahlke is an employee of Impulze GmbH Zürich and provides consultancy services for F. Hoffmann-La Roche Ltd. T. Coetzee is an employee of the National Multiple Sclerosis Society USA. G.R. Cutter has served on the following data and safety monitoring boards: AI Therapeutics, AMO Pharma, AstraZeneca, Avexis Pharmaceuticals, Biolinerx, Brainstorm Cell Therapeutics, Bristol Myers Squibb/Celgene, CSL Behring, Galmed Pharmaceuticals, Green Valley Pharma, Horizon Pharmaceuticals, Immunic, Mapi Pharmaceuticals Ltd, Merck, Mitsubishi Tanabe Pharma Holdings, Opko Biologics, Prothena Biosciences, Novartis, Regeneron, Sanofi-Aventis, Reata Pharmaceuticals, NHLBI (Protocol Review Committee), University of Texas Southwestern, University of Pennsylvania, and Visioneering Technologies Inc.; has served on the following consulting or advisory boards: Alexion, Antisense Therapeutics, Biogen, Clinical Trial Solutions LLC, Genzyme, Genentech Inc., GW Pharmaceuticals, Immunic, Klein-Buendel Inc., Merck-Serono, Novartis, Osmotica Pharmaceuticals, Perception Neurosciences, Protalix Biotherapeutics, Recursion/Cerexis Pharmaceuticals, Regeneron, Roche, and SAB Biotherapeutics; is employed by the University of Alabama at Birmingham; and is president of Pythagoras Inc., a private consulting company located in Birmingham, AL. S. Yuen is an employee of Genentech Inc., and a shareholder in F. Hoffmann-La Roche Ltd. U. Bonati is an employee of and a shareholder in F. Hoffmann-La Roche Ltd. F.D. Lublin has participated in consulting agreements/advisory boards/DSMB for Biogen, EMD Serono, Novartis, Teva, Actelion/Janssen, Sanofi/Genzyme, Acorda, Roche/Genentech, Viela Bio/Horizon Therapeutics, Receptos/Celgene/Bristol Myers Squibb, Mapi Pharma, Brainstorm Cell Therapeutics, Jazz Pharma, Mylan, Immunic, Population Council, Avotres, Neurogene, Banner Life Sciences, LabCorp, Entelexo Biotherapeutics, Neuralight, SetPoint Medical, Hexal/Sandoz, Baim Institute, Sudo Biosciences, and Lapix Therapeutics; has stock options in Avotres and Neuralight; and has served as a speaker for Sanofi and Biogen. Go to
Figures
Comment in
-
A Newly Validated Gold Standard for Measuring Multiple Sclerosis Disability Worsening.Neurology. 2025 May 27;104(10):e213672. doi: 10.1212/WNL.0000000000213672. Epub 2025 Apr 23. Neurology. 2025. PMID: 40266716 No abstract available.
References
-
- Kapoor R, Ho P-R, Campbell N, et al. . Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018;17(5):405-415. doi:10.1016/S1474-4422(18)30069-3 - DOI - PubMed
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
