Statistical signature of subtle behavioral changes in large-scale assays
- PMID: 40258220
- PMCID: PMC12121925
- DOI: 10.1371/journal.pcbi.1012990
Statistical signature of subtle behavioral changes in large-scale assays
Abstract
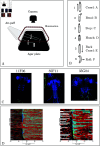
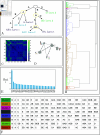
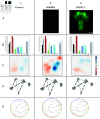
The central nervous system can generate various behaviors, including motor responses, which we can observe through video recordings. Recent advances in gene manipulation, automated behavioral acquisition at scale, and machine learning enable us to causally link behaviors to their underlying neural mechanisms. Moreover, in some animals, such as the Drosophila melanogaster larva, this mapping is possible at the unprecedented scale of single neurons, allowing us to identify the neural microcircuits generating particular behaviors. These high-throughput screening efforts, linking the activation or suppression of specific neurons to behavioral patterns in millions of animals, provide a rich dataset to explore the diversity of nervous system responses to the same stimuli. However, important challenges remain in identifying subtle behaviors, including immediate and delayed responses to neural activation or suppression, and understanding these behaviors on a large scale. We here introduce several statistically robust methods for analyzing behavioral data in response to these challenges: 1) A generative physical model that regularizes the inference of larval shapes across the entire dataset. 2) An unsupervised kernel-based method for statistical testing in learned behavioral spaces aimed at detecting subtle deviations in behavior. 3) A generative model for larval behavioral sequences, providing a benchmark for identifying higher-order behavioral changes. 4) A comprehensive analysis technique using suffix trees to categorize genetic lines into clusters based on common action sequences. We showcase these methodologies through a behavioral screen focused on responses to an air puff, analyzing data from 280 716 larvae across 569 genetic lines.
Copyright: © 2025 Blanc et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Discovery of brainwide neural-behavioral maps via multiscale unsupervised structure learning.Science. 2014 Apr 25;344(6182):386-92. doi: 10.1126/science.1250298. Epub 2014 Mar 27. Science. 2014. PMID: 24674869
-
Switch-like and persistent memory formation in individual Drosophila larvae.Elife. 2021 Oct 12;10:e70317. doi: 10.7554/eLife.70317. Elife. 2021. PMID: 34636720 Free PMC article.
-
The serotonergic central nervous system of the Drosophila larva: anatomy and behavioral function.PLoS One. 2012;7(10):e47518. doi: 10.1371/journal.pone.0047518. Epub 2012 Oct 17. PLoS One. 2012. PMID: 23082175 Free PMC article.
-
Quantifying behavior to solve sensorimotor transformations: advances from worms and flies.Curr Opin Neurobiol. 2017 Oct;46:90-98. doi: 10.1016/j.conb.2017.08.006. Epub 2017 Aug 30. Curr Opin Neurobiol. 2017. PMID: 28850885 Free PMC article. Review.
-
Seeing the light: photobehavior in fruit fly larvae.Trends Neurosci. 2012 Feb;35(2):104-10. doi: 10.1016/j.tins.2011.11.003. Epub 2012 Jan 4. Trends Neurosci. 2012. PMID: 22222349 Review.
Cited by
-
Feeding state-dependent neuropeptidergic modulation of reciprocally interconnected inhibitory neurons biases sensorimotor decisions in Drosophila.Nat Commun. 2025 Sep 2;16(1):8198. doi: 10.1038/s41467-025-61805-y. Nat Commun. 2025. PMID: 40897720 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

