Endogenous acrolein accumulation in akr7a3 mutants causes microvascular dysfunction due to increased arachidonic acid metabolism
- PMID: 40258306
- PMCID: PMC12051060
- DOI: 10.1016/j.redox.2025.103639
Endogenous acrolein accumulation in akr7a3 mutants causes microvascular dysfunction due to increased arachidonic acid metabolism
Abstract
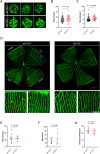
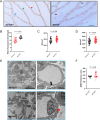
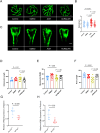
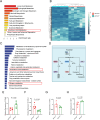
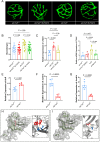
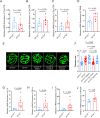
Acrolein (ACR) is an endogenous reactive unsaturated aldehyde that can be detoxified by the aldo-keto reductase (AKR) enzyme system. While it has been shown that accumulation of ACR is associated with several health problems, including inflammation, oxidative stress, and cardiovascular disease the study aimed to analyze whether an endogenous accumulation of ACR is causal for vascular dysfunction in an akr7a3 mutant zebrafish model. Enlargement of the hyaloid and retinal vasculature, as well as alterations in the larval pronephros and thickening of the glomerular basement membrane in the adult kidney were found upon ACR accumulation. Transcriptomic and metabolomic analyses, followed by functional validation, revealed that the up-regulation of genes controlling the arachidonic acid metabolism and activation of the leukotriene pathway are responsible for the observed microvascular changes. In conclusion, the data have identified an intrinsic function of ACR in akr7a3 mutants that activates the arachidonic acid metabolism and subsequently disrupts vascular integrity by promoting an inflammatory response. Thus, ACR is causal in the development of vascular disease.
Keywords: Acrolein; Aldo-keto reductase; Arachidonic acid metabolism; Kidney alteration; Ocular vascular diseases; Zebrafish.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation.Health Technol Assess. 2006 Sep;10(31):iii-iv, xiii-xvi, 1-239. doi: 10.3310/hta10310. Health Technol Assess. 2006. PMID: 16948890
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Minocycline for acne vulgaris: efficacy and safety.Cochrane Database Syst Rev. 2003;(1):CD002086. doi: 10.1002/14651858.CD002086. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2012 Aug 15;(8):CD002086. doi: 10.1002/14651858.CD002086.pub2. PMID: 12535427 Updated.
-
A systematic overview of chemotherapy effects in acute myeloid leukaemia.Acta Oncol. 2001;40(2-3):231-52. doi: 10.1080/02841860151116321. Acta Oncol. 2001. PMID: 11441935
-
Chemotherapy for advanced gastric cancer.Cochrane Database Syst Rev. 2017 Aug 29;8(8):CD004064. doi: 10.1002/14651858.CD004064.pub4. Cochrane Database Syst Rev. 2017. PMID: 28850174 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

