Electrophysiological Correlates of Lucid Dreaming: Sensor and Source Level Signatures
- PMID: 40258661
- PMCID: PMC12079745
- DOI: 10.1523/JNEUROSCI.2237-24.2025
Electrophysiological Correlates of Lucid Dreaming: Sensor and Source Level Signatures
Abstract
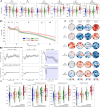
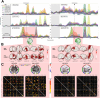
Lucid dreaming (LD) is a state of conscious awareness of the ongoing oneiric state, predominantly linked to REM sleep. Progress in understanding its neurobiological basis has been hindered by small sample sizes, diverse EEG setups, and artifacts like saccadic eye movements. To address these challenges in characterizing the electrophysiological correlates of LD, we introduced an adaptive multistage preprocessing pipeline, applied to human data (male and female) pooled across laboratories, allowing us to explore sensor- and source-level markers of LD. We observed that, while sensor-level differences between LD and nonlucid REM sleep were minimal, mixed-frequency analysis revealed broad low alpha to gamma power reductions during LD compared with wakefulness. Source-level analyses showed significant beta power (12-30 Hz) reductions in right central and parietal areas, including the temporoparietal junction, during LD. Moreover, functional connectivity in the alpha band (8-12 Hz) increased during LD compared with nonlucid REM sleep. During initial LD eye signaling compared with the baseline, source-level gamma1 power (30-36 Hz) increased in right temporo-occipital regions, including the right precuneus. Finally, functional connectivity analysis revealed increased interhemispheric and inter-regional gamma1 connectivity during LD, reflecting widespread network engagement. These results suggest that distinct source-level power and connectivity patterns characterize the dynamic neural processes underlying LD, including shifts in network communication and regional activation that may underlie the specific changes in perception, memory processing, self-awareness, and cognitive control. Taken together, these findings illuminate the electrophysiological correlates of LD, laying the groundwork for decoding the mechanisms of this intriguing state of consciousness.
Keywords: REM sleep; consciousness; dreams; lucid dream; metacognition; self-awareness.
Copyright © 2025 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Appelhoff S, Hurst AJ, Lawrence A, Li A, Ramos M, José Y, O’Reilly C, Xiang L, Jonte D (2022) PyPREP: a Python implementation of the preprocessing pipeline (PREP) for EEG data (Version 0.4.2) [Computer software]. 10.5281/zenodo.6363576 - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
