Age-related divergence of circulating immune responses in patients with solid tumors treated with immune checkpoint inhibitors
- PMID: 40258833
- PMCID: PMC12012091
- DOI: 10.1038/s41467-025-58512-z
Age-related divergence of circulating immune responses in patients with solid tumors treated with immune checkpoint inhibitors
Abstract
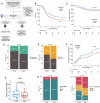
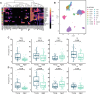
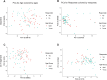
Most new cancer diagnoses occur in patients over the age of 65. The composition and function of the immune system changes with age, but how the aged immune system affects responses to immune checkpoint inhibitor (ICI) cancer therapies remains incompletely understood. Here, using multiplex cytokine assay and high-parameter mass cytometry, we analyze prospectively collected blood samples from 104 cancer patients receiving ICIs. We find aged patients ( ≥ 65-years-old; n = 54) derive similar clinical outcomes as younger patients (n = 50). However, aged, compared to young, patients have divergent immune phenotypes at baseline that persist during ICI therapy, including diminished cytokine responses, reduced pools of naïve T cells with increased relative expression of immune checkpoint molecules, and more robust effector T cell expansion in responders compared to non-responders. Our study provides insights into age-stratified mechanisms of ICI effects while also implying the utility of age-tailored immunotherapeutic approaches.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: E.J.L. reports the following: Consultant/Advisor: Agenus, Bristol-Myers Squibb, CareDx, Eisai, Genentech, HUYA Bioscience International, Immunocore, Instil Bio, IO Biotech, Lyvgen, Merck, Merck KGaA, Natera, Nektar, Novartis, OncoSec, Pfizer, Rain Therapeutics, Regeneron, Replimune, Sanofi-Aventis, Sun Pharma, Syneos Health. Institutional Research Funding: 1104Health, Bristol-Myers Squibb, Haystack Oncology, Merck, Regeneron, Sanofi. Stock: Iovance. E.J.L is supported by Bloomberg~Kimmel Institute for Cancer Immunotherapy, the Marilyn and Michael Glosserman Fund for Basal Cell Carcinoma and Melanoma Research, the Barney Family Foundation, and the Laverna Hahn Charitable Trust. M.B. reports Grant/Research Support (paid to Johns Hopkins): Merck and Consulting: Exelixis, Incyte, AstraZeneca. E.M.J. reports other support from Abmeta and Adventris, personal fees from Dragonfly, Neuvogen, Surge Tx, Mestag, HDTbio, and grants from Lustgarten, Genentech, BMS, NeoTx, and Break Through Cancer. Dr. Elizabeth Jaffee is a founder of and holds equity in Adventris Pharmaceuticals. She also serves as a consultant to the entity. Further, Adventris Pharmaceuticals has licensed a technology described in this study from the Johns Hopkins University. As a result of that agreement, Dr. Jaffee and the University are entitled to royalty distributions related to technology described in the study discussed in this publication. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. G.S.C. is an employee and stockholder of Roche. G.S.C. received support for preparation of this manuscript and corresponding travel from Roche. G.S.C. is a co-inventor on patents filed by Genentech/Roche that are related to atezolizumab use. R.M. is an employee and stockholder of F. Hoffman-La Roche, Ltd. R.M. is a co-inventor on patents filed by Genentech/Roche that are related to atezolizumab use. W.J.H. reports patent royalties from Rodeo/Amgen; grants from Sanofi, NeoTX, and Riboscience; speaking/travel honoraria from Exelixis and Standard BioTools. M.Y. has received grant/research support (to Johns Hopkins) from Bristol-Myers Squibb, Incyte and Genentech/Roche; has received honoraria from Genentech/Roche, Exelixis, AstraZeneca, Replimune, Hepion, and Lantheus; and is a cofounder with equity of Adventris. D.J.Z. reports grant support (to Johns Hopkins) and travel from Roche/Genentech, and honoraria from Omni Health Media, Sermo, Atheneum, Escientiq, Deerfield Institute, ZoomRx, and NeuCore Bio. The remaining authors declare no competing interests.
Figures



References
-
- Cancer of Any Site - Cancer Stat Facts. SEERhttps://seer.cancer.gov/statfacts/html/all.html.
-
- Pallis, A. G. et al. EORTC elderly task force position paper: Approach to the older cancer patient. Eur. J. Cancer46, 1502–1513 (2010). - PubMed
-
- Repetto, L. Greater risks of chemotherapy toxicity in elderly patients with cancer. J. Support. Oncol.1, 18–24 (2003). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

