Characterization of neutralizing versus binding antibody and T cell responses to varicella-zoster virus in the elderly
- PMID: 40258885
- PMCID: PMC12012113
- DOI: 10.1038/s41598-025-98107-8
Characterization of neutralizing versus binding antibody and T cell responses to varicella-zoster virus in the elderly
Abstract
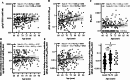
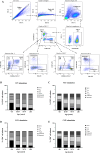
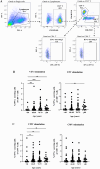
Age-related immune changes increase the risk of herpes zoster (HZ) caused by varicella-zoster virus (VZV) reactivation. Understanding immune responses to VZV is crucial for reducing the burden of HZ in aging populations. Due to the limited availability of data regarding the VZV immune profiles of elderly individuals, particularly in developing countries, more comprehensive immunological investigations are warranted. A total of 213 participants aged ≥ 60 years were included in this study. VZV-neutralizing antibodies (NAb) and glycoprotein-binding antibodies (BAb) were quantified. Furthermore, VZV-specific T cell subsets and their functionality were evaluated using flow cytometry. Elderly individuals demonstrated a high VZV seropositivity rate of 98.6%, exceeding that of the younger adults. Interestingly, VZV-BAb increased, whereas the proportion of NAb decreased with age, with a significantly lower proportion in the elderly aged ≥ 70 years. The elderly showed decreased naïve T cells and accumulated VZV-specific aged T cells; central memory and effector memory CD4+ and CD8+ T cells, and terminal effector memory CD8+ T cells; with elevated expression of senescence and exhaustion markers, indicating functional impairment. Nonetheless, VZV-specific functional T cells; percentages of VZV-specific interferon-γ-secreting CD4+ and CD8+ T cells; were not diminished. These findings provide insights into aging VZV immune profiles, which will facilitate the development of age-specific HZ vaccination policies.
Keywords: Aging; Immunosenescence; Shingles; Varicella-zoster virus.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Breadth and Functionality of Varicella-Zoster Virus Glycoprotein-Specific Antibodies Identified after Zostavax Vaccination in Humans.J Virol. 2018 Jun 29;92(14):e00269-18. doi: 10.1128/JVI.00269-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29743372 Free PMC article.
-
Varicella-Zoster Virus-Specific Cellular Immune Responses to the Live Attenuated Zoster Vaccine in Young and Older Adults.J Immunol. 2017 Jul 15;199(2):604-612. doi: 10.4049/jimmunol.1700290. Epub 2017 Jun 12. J Immunol. 2017. PMID: 28607114 Free PMC article.
-
Systemic varicella zoster virus reactive effector memory T-cells impaired in the elderly and in kidney transplant recipients.J Med Virol. 2012 Dec;84(12):2018-25. doi: 10.1002/jmv.23427. J Med Virol. 2012. PMID: 23080511
-
VZV T cell-mediated immunity.Curr Top Microbiol Immunol. 2010;342:341-57. doi: 10.1007/82_2010_31. Curr Top Microbiol Immunol. 2010. PMID: 20473790 Review.
-
Varicella zoster virus vasculopathy: clinical features and pathogenesis.J Neurovirol. 2014 Apr;20(2):157-63. doi: 10.1007/s13365-013-0183-9. Epub 2013 Aug 6. J Neurovirol. 2014. PMID: 23918503 Free PMC article. Review.
Cited by
-
A novel adjuvant system BK-02 with CpG2006 and MF59 enhances the immunogenicity of a herpes zoster subunit vaccine.Front Immunol. 2025 Jul 15;16:1641109. doi: 10.3389/fimmu.2025.1641109. eCollection 2025. Front Immunol. 2025. PMID: 40735325 Free PMC article.
References
-
- Muñoz-Quiles, C., López-Lacort, M., Díez-Domingo, J. & Orrico-Sánchez, A. Herpes Zoster risk and burden of disease in immunocompromised populations: a population-based study using health system integrated databases, 2009–2014. BMC Infect. Dis.20, 905. 10.1186/s12879-020-05648-6 (2020). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

