Enhanced peri-implantitis management through purple-LED irradiation coupled with silver ion application and calcium phosphate gene transfection carrier coating
- PMID: 40258901
- PMCID: PMC12012141
- DOI: 10.1038/s41598-025-96075-7
Enhanced peri-implantitis management through purple-LED irradiation coupled with silver ion application and calcium phosphate gene transfection carrier coating
Abstract
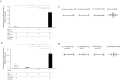
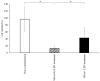
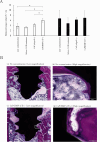
The aim of this study was to investigate the bactericidal effect and recovery of biocompatibility of contaminated titanium surfaces using a combination treatment involving silver, copper, or iron ion application along with 400 nm purple-LED light irradiation. Additionally, the study sought to develop a functional calcium phosphate (CaP) coating treatment on titanium surfaces following disinfection, to promote re-osseointegration. A purple-LED emitting light at 400 nm was utilized to irradiate Staphylococcus aureus suspensions and biofilms in the presence of various concentrations of silver, copper, and iron solutions for 1 min. The bactericidal effect and electron spin resonance (ESR) spectrum were subsequently evaluated. Additionally, the hydrophilicity of the titanium surface and cell viability of MC3T3-E1 cells after combination treatment with silver ion was evaluated. Furthermore, a titanium surface coating with CaP gene transfection carrier containing plasmid DNA was developed using an electric current. The activity of hard tissue formation was then evaluated both in vitro and in vivo post-treatment. The bactericidal effect of the combination treatment with silver ions was attributed to the generation of hydroxyl radicals, whereas the effects from iron and copper treatments were not radical-mediated. The silver treatment significantly restored the hydrophilicity and cell affinity of the titanium surface. Moreover, CaP coating applied via an electric current (30 µA for 5 min) enhanced hard tissue formation activity on the titanium surface in both in vitro and in vivo settings. The combination treatment utilizing silver ions and purple-LED irradiation significantly enhanced bactericidal effects by generating high levels of hydroxyl radicals. Additionally, coating the titanium surface with functionalized CaP promoted early osseointegration, suggesting a promising strategy for improving implant outcomes.
Keywords: Calcium phosphate; Gene transfection; Hydroxyl radicals; Purple LED; Silver; Titanium surface.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics statement: All methods are reported in accordance with ARRIVE guidelines ( https://arriveguidelines.org ).
Figures




Similar articles
-
Bactericidal activity and recovery effect of hydroxyl radicals generated by ultraviolet irradiation and silver ion application on an infected titanium surface.Sci Rep. 2020 May 22;10(1):8553. doi: 10.1038/s41598-020-65411-4. Sci Rep. 2020. PMID: 32444858 Free PMC article.
-
Biofunctionalization of selective laser melted porous titanium using silver and zinc nanoparticles to prevent infections by antibiotic-resistant bacteria.Acta Biomater. 2020 Apr 15;107:325-337. doi: 10.1016/j.actbio.2020.02.044. Epub 2020 Mar 4. Acta Biomater. 2020. PMID: 32145392
-
Synthesis of new antibacterial composite coating for titanium based on highly ordered nanoporous silica and silver nanoparticles.Mater Sci Eng C Mater Biol Appl. 2014 Dec;45:146-53. doi: 10.1016/j.msec.2014.08.057. Epub 2014 Sep 4. Mater Sci Eng C Mater Biol Appl. 2014. PMID: 25491813
-
Nanostructured Ag+-substituted fluorhydroxyapatite-TiO2 coatings for enhanced bactericidal effects and osteoinductivity of Ti for biomedical applications.Int J Nanomedicine. 2018 May 3;13:2665-2684. doi: 10.2147/IJN.S162558. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29760549 Free PMC article.
-
Enhanced silver loaded antibacterial titanium implant coating with novel hierarchical effect.J Biomater Appl. 2018 Apr;32(9):1289-1299. doi: 10.1177/0885328218755538. Epub 2018 Feb 8. J Biomater Appl. 2018. PMID: 29417864
References
-
- Hasuike, et al. Evidence and future challenges for diagnosis, risk factors, and treatment of peri-implantitis. J. Japan. Assoc. Periodontol.65, 81–92 (2023).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

