The primary somatosensory sensory cortex-basolateral amygdala pathway contributes to comorbid depression in spared nerve injury-induced neuropathic pain
- PMID: 40258918
- PMCID: PMC12012082
- DOI: 10.1038/s41598-025-97164-3
The primary somatosensory sensory cortex-basolateral amygdala pathway contributes to comorbid depression in spared nerve injury-induced neuropathic pain
Abstract
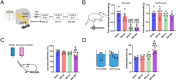
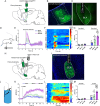
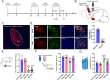
Comorbid depression in chronic pain is a prevalent health problem, yet the underlying neural mechanisms remain largely unexplored. This study identified a dedicated neural circuit connecting the hind limb region of the primary somatosensory cortex (S1HL) to the basolateral amygdala (BLA) that mediated neuropathic pain-induced depression. We demonstrated that depressive-like behaviors in the chronic phase of a mouse neuropathic pain model were associated with heightened activity in the S1HL and BLA. Using viral tracing and RNAscope in situ hybridization, we characterized the circuit architecture of S1HL glutamatergic projections to BLA cholecystokinin (CCK) neurons (S1HLGlu → BLACCK). In vivo fiber photometry calcium imaging revealed that both the S1HL BLA-projecting afferents and the BLA S1HL-innervating neurons exhibited hyperactivity in neuropathic pain-induced depressive states. Chemogenetic inhibition of the S1HL → BLA circuit could block neuropathic pain-induced depressive-like behaviors. In addition, specific knockdown of CCK expression in BLA S1HL-innervating neurons alleviated these depressive-like behaviors. Our findings demonstrated that the cortical-amygdala circuit S1HLGlu → BLACCK drove the transition from chronic pain to depression, thus suggesting a potential neural circuit basis for treating chronic pain-related depressive disorders.
Keywords: Basolateral amygdala; Cholecystokinin; Chronic pain; Depression; Primary somatosensory cortex.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Infralimbic-basolateral amygdala circuit associated with depression-like not anxiety-like behaviors induced by chronic neuropathic pain and the antidepressant effects of electroacupuncture.Brain Res Bull. 2024 Nov;218:111092. doi: 10.1016/j.brainresbull.2024.111092. Epub 2024 Oct 5. Brain Res Bull. 2024. PMID: 39369764
-
Mapping the pain pathway: The VPL-S1HL-ACC circuit's role in central post-stroke pain.Brain Res Bull. 2025 Jul;227:111406. doi: 10.1016/j.brainresbull.2025.111406. Epub 2025 May 29. Brain Res Bull. 2025. PMID: 40449628
-
Contribution of AMPA Receptor-Mediated LTD in LA/BLA-CeA Pathway to Comorbid Aversive and Depressive Symptoms in Neuropathic Pain.J Neurosci. 2021 Aug 25;41(34):7278-7299. doi: 10.1523/JNEUROSCI.2678-20.2021. Epub 2021 Jul 16. J Neurosci. 2021. PMID: 34272314 Free PMC article.
-
A systematic review of the pain-related emotional and cognitive impairments in chronic inflammatory pain induced by CFA injection and its mechanism.IBRO Neurosci Rep. 2025 Feb 27;18:414-431. doi: 10.1016/j.ibneur.2025.02.015. eCollection 2025 Jun. IBRO Neurosci Rep. 2025. PMID: 40124113 Free PMC article. Review.
-
Chronic Neuropathic Pain and Comorbid Depression Syndrome: From Neural Circuit Mechanisms to Treatment.ACS Chem Neurosci. 2024 Jul 3;15(13):2432-2444. doi: 10.1021/acschemneuro.4c00125. Epub 2024 Jun 25. ACS Chem Neurosci. 2024. PMID: 38916052 Review.
References
-
- Cohen, S. P., Vase, L. & Hooten, W. M. Chronic pain: an update on burden, best practices, and new advances. Lancet397, 2082–2097. 10.1016/s0140-6736(21)00393-7 (2021). - PubMed
-
- Malhi, G. S. & Mann, J. J. Depression. Lancet392, 2299–2312. 10.1016/s0140-6736(18)31948-2 (2018). - PubMed
-
- Emptage, N. P., Sturm, R. & Robinson, R. L. Depression and comorbid pain as predictors of disability, employment, insurance status, and health care costs. Psychiatr Serv56, 468–474. 10.1176/appi.ps.56.4.468 (2005). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

