Mitochondrial NADPH fuels mitochondrial fatty acid synthesis and lipoylation to power oxidative metabolism
- PMID: 40258949
- PMCID: PMC12331256
- DOI: 10.1038/s41556-025-01655-4
Mitochondrial NADPH fuels mitochondrial fatty acid synthesis and lipoylation to power oxidative metabolism
Erratum in
-
Author Correction: Mitochondrial NADPH fuels mitochondrial fatty acid synthesis and lipoylation to power oxidative metabolism.Nat Cell Biol. 2025 Jul;27(7):1200. doi: 10.1038/s41556-025-01695-w. Nat Cell Biol. 2025. PMID: 40404895 No abstract available.
Abstract
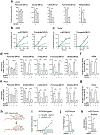
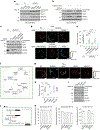
Nicotinamide adenine dinucleotide phosphate (NADPH) is a vital electron donor essential for macromolecular biosynthesis and protection against oxidative stress. Although NADPH is compartmentalized within the cytosol and mitochondria, the specific functions of mitochondrial NADPH remain largely unexplored. Here we demonstrate that NAD+ kinase 2 (NADK2), the principal enzyme responsible for mitochondrial NADPH production, is critical for maintaining protein lipoylation, a conserved lipid modification necessary for the optimal activity of multiple mitochondrial enzyme complexes, including the pyruvate dehydrogenase complex. The mitochondrial fatty acid synthesis (mtFAS) pathway utilizes NADPH for generating protein-bound acyl groups, including lipoic acid. By developing a mass-spectrometry-based method to assess mammalian mtFAS, we reveal that NADK2 is crucial for mtFAS activity. NADK2 deficiency impairs mtFAS-associated processes, leading to reduced cellular respiration and mitochondrial translation. Our findings support a model in which mitochondrial NADPH fuels the mtFAS pathway, thereby sustaining protein lipoylation and mitochondrial oxidative metabolism.
© 2025. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures








References
MeSH terms
Substances
Grants and funding
- R01 GM143236/GM/NIGMS NIH HHS/United States
- RP240035/Cancer Prevention and Research Institute of Texas (Cancer Prevention Research Institute of Texas)
- F30 DK137407/DK/NIDDK NIH HHS/United States
- 00036920/Pew Charitable Trusts
- 3R01GM143236-02S1/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- I-2067-20240404/Welch Foundation
- 2021-67013-33778/United States Department of Agriculture | Agricultural Research Service (USDA Agricultural Research Service)
- R01GM143236/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- P30 CA142543/CA/NCI NIH HHS/United States
- R01 CA289251/CA/NCI NIH HHS/United States
- RR190087/Cancer Prevention and Research Institute of Texas (Cancer Prevention Research Institute of Texas)
- S10OD030312-01/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- RSG-22-177-01-TBE/American Cancer Society (American Cancer Society, Inc.)
- V2021-019/V Foundation for Cancer Research (V Foundation)
- 1R01CA289251-01A1/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- RP210041/Cancer Prevention and Research Institute of Texas (Cancer Prevention Research Institute of Texas)
- DK137407/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
LinkOut - more resources
Full Text Sources

