Lysosomal EGFR acts as a Rheb-GEF independent of its kinase activity to activate mTORC1
- PMID: 40259053
- PMCID: PMC12205066
- DOI: 10.1038/s41422-025-01110-x
Lysosomal EGFR acts as a Rheb-GEF independent of its kinase activity to activate mTORC1
Abstract
Oncogenic mutations in EGFR often result in EGF-independent constitutive activation and aberrant trafficking and are associated with several human malignancies, including non-small cell lung cancer. A major consequence of EGFR mutations is the activation of the mechanistic target of rapamycin complex 1 (mTORC1), which requires EGFR kinase activity and downstream PI3K/AKT signaling, resulting in increased cell proliferation. However, recent studies have elucidated kinase-independent roles of EGFR in cell survival and cancer progression. Here, we report a cis mTORC1 activation function of EGFR that is independent of its kinase activity. Our results reveal that lysosomal localization of EGFR is critical to mTORC1 activation, where EGFR physically binds Rheb, acting as a guanine exchange factor (GEF) for Rheb, with its Glu804 serving as a potential glutamic finger. Genetic knock-in of EGFR-E804K in cells reduces the level of GTP-bound Rheb, and significantly suppresses mTORC1 activation, cell proliferation and tumor growth. Different tyrosine kinase inhibitors exhibit distinct effects on EGFR-induced mTORC1 activation, with afatinib, which additionally blocks EGFR's GEF activity, causing a much greater suppression of mTORC1 activation and cell growth, and erlotinib, which targets only kinase activity, resulting in only a slight decrease. Moreover, a novel small molecule, BIEGi-1, was designed to target both the Rheb-GEF and kinase activities of EGFR, and shows a strong inhibitory effect on the viability of cells harboring EGFR mutants. These findings unveil a fundamental event in cell growth and suggest a promising strategy against cancers with EGFR mutations.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: K.D. and F.Z. are co-inventors of the patent WO/2014/079232. The authors declare no other competing interests.
Figures

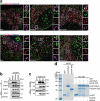
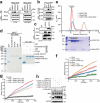



References
-
- Battaglioni, S., Benjamin, D., Walchli, M., Maier, T. & Hall, M. N. mTOR substrate phosphorylation in growth control. Cell185, 1814–1836 (2022). - PubMed
-
- Kim, J. & Guan, K. L. mTOR as a central hub of nutrient signalling and cell growth. Nat. Cell Biol.21, 63–71 (2019). - PubMed
-
- Valvezan, A. J. & Manning, B. D. Molecular logic of mTORC1 signalling as a metabolic rheostat. Nat. Metab.1, 321–333 (2019). - PubMed
MeSH terms
Substances
Grants and funding
- 82030090/National Natural Science Foundation of China (National Science Foundation of China)
- 82341015/National Natural Science Foundation of China (National Science Foundation of China)
- 32170710/National Natural Science Foundation of China (National Science Foundation of China)
- 82173098/National Natural Science Foundation of China (National Science Foundation of China)
- 32320103002/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

