Impact of myelodysplasia-related gene mutations and residual mutations at remission in venetoclax/azacitidine for AML
- PMID: 40259101
- PMCID: PMC12133561
- DOI: 10.1038/s41375-025-02625-3
Impact of myelodysplasia-related gene mutations and residual mutations at remission in venetoclax/azacitidine for AML
Abstract
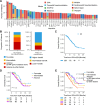
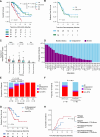
Venetoclax plus azacitidine (VEN + AZA) is widely used in acute myeloid leukemia (AML). This study explored the role of static and dynamic profiles of mutational clonal burden to predict outcomes by analyzing marrow samples from 228 VEN + AZA treated AML cases at "Pre-treatment" (n = 228), "Best-response" (n = 105), and "Relapse" (n = 27) phases using targeted-capture sequencing. In a multivariate model, older age, prior AZA, TP53 mutation with variant allele frequency ≥0.10, and RAS-pathway mutations predicted shorter overall survival (OS), while BCORL1 mutation predicted longer OS. Notably, myelodysplasia-related gene mutations, which constitute adverse factors in ELN 2022, predicted favorable survival. Achieving composite complete remission (CRc) significantly predicted longer OS (P < 0.001) but showed residual mutations in 76.2% of the cases. Among CRc cases, relapse-free survival was stratified by molecular clearance of mutations other than DNMT3A, ASXL1, and TET2 (P = 0.04). In addition, 37% of relapsed cases showed a change of major clones, with 40% having potential targets of molecular-targeting treatment. This study revealed the novel prognostic role of myelodysplasia-related gene mutations and established the importance of molecular response assessment in CRc phase.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: YI received honoraria from Nippon Shinyaku Co., Ltd. and AbbVie GK. NN received honoraria from Nippon Shinyaku Co., Ltd. and AbbVie GK. YK received honoraria from AbbVie GK. NH received honoraria from Nippon Shinyaku Co., Ltd. and AbbVie GK. J. Kanda received honoraria from Nippon Shinyaku Co., Ltd. and AbbVie GK. YO received research funding and honoraria from Nippon Shinyaku Co., Ltd. SC received honoraria from Nippon Shinyaku Co., Ltd. M.Ichii received honoraria from Nippon Shinyaku Co., Ltd. KI received honoraria from Nippon Shinyaku Co., Ltd. and AbbVie GK. SY received honoraria from Nippon Shinyaku Co., Ltd. and AbbVie GK. KM received honoraria from Nippon Shinyaku Co., Ltd. and AbbVie GK. T. Kitano received honoraria from Nippon Shinyaku Co., Ltd. SK received honoraria from AbbVie GK. AT received honoraria from Nippon Shinyaku Co., Ltd. and AbbVie GK. NK received honoraria from AbbVie GK. S.Ogawa received research funding from Nippon Shinyaku Co., Ltd. YN received honoraria from Nippon Shinyaku Co., Ltd. and AbbVie GK.
Figures
References
-
- DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383:617–29. - PubMed
-
- DiNardo CD, Wei AH. How I treat acute myeloid leukemia in the era of new drugs. Blood. 2020;135:85–96. - PubMed
-
- Pollyea DA, Pratz K, Letai A, Jonas BA, Wei AH, Pullarkat V, et al. Venetoclax with azacitidine or decitabine in patients with newly diagnosed acute myeloid leukemia: long term follow-up from a phase 1b study. Am J Hematol. 2021;96:208–17. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

