Scaffold-free endocrine tissue engineering: role of islet organization and implications in type 1 diabetes
- PMID: 40259265
- PMCID: PMC12010671
- DOI: 10.1186/s12902-025-01919-y
Scaffold-free endocrine tissue engineering: role of islet organization and implications in type 1 diabetes
Abstract
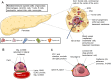
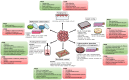
Type 1 diabetes (T1D) is a chronic hyperglycemia disorder emerging from beta-cell (insulin secreting cells of the pancreas) targeted autoimmunity. As the blood glucose levels significantly increase and the insulin secretion is gradually lost, the entire body suffers from the complications. Although various advances in the insulin analogs, blood glucose monitoring and insulin application practices have been achieved in the last few decades, a cure for the disease is not obtained. Alternatively, pancreas/islet transplantation is an attractive therapeutic approach based on the patient prognosis, yet this treatment is also limited mainly by donor shortage, life-long use of immunosuppressive drugs and risk of disease transmission. In research and clinics, such drawbacks are addressed by the endocrine tissue engineering of the pancreas. One arm of this engineering is scaffold-free models which often utilize highly developed cell-cell junctions, soluble factors and 3D arrangement of islets with the cellular heterogeneity to prepare the transplant formulations. In this review, taking T1D as a model autoimmune disease, techniques to produce so-called pseudoislets and their applications are studied in detail with the aim of understanding the role of mimicry and pointing out the promising efforts which can be translated from benchside to bedside to achieve exogenous insulin-free patient treatment. Likewise, these developments in the pseudoislet formation are tools for the research to elucidate underlying mechanisms in pancreas (patho)biology, as platforms to screen drugs and to introduce immunoisolation barrier-based hybrid strategies.
Keywords: Beta-cell models; Beta-cell replacement therapy; Cell-cell interactions; Diabetes; Drug screening; Pancreas (patho)biology; Pseudoislet.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Bioengineering of a human iPSC-derived vascularized endocrine pancreas for type 1 diabetes.Cell Rep Med. 2025 Feb 18;6(2):101938. doi: 10.1016/j.xcrm.2025.101938. Epub 2025 Feb 7. Cell Rep Med. 2025. PMID: 39922198 Free PMC article.
-
Islet alloautotransplantation: Allogeneic pancreas transplantation followed by transplant pancreatectomy and islet transplantation.Am J Transplant. 2018 Apr;18(4):1016-1019. doi: 10.1111/ajt.14593. Epub 2017 Dec 18. Am J Transplant. 2018. PMID: 29160954
-
Bioengineered human pseudoislets form efficiently from donated tissue, compare favourably with native islets in vitro and restore normoglycaemia in mice.Diabetologia. 2018 Sep;61(9):2016-2029. doi: 10.1007/s00125-018-4672-5. Epub 2018 Jul 3. Diabetologia. 2018. PMID: 29971529 Free PMC article.
-
Recreating the Endocrine Niche: Advances in Bioengineering the Pancreas.Artif Organs. 2025 Apr;49(4):541-555. doi: 10.1111/aor.14950. Epub 2025 Jan 23. Artif Organs. 2025. PMID: 39844747 Review.
-
Restoring normal islet mass and function in type 1 diabetes through regenerative medicine and tissue engineering.Lancet Diabetes Endocrinol. 2021 Oct;9(10):708-724. doi: 10.1016/S2213-8587(21)00170-4. Epub 2021 Sep 1. Lancet Diabetes Endocrinol. 2021. PMID: 34480875 Free PMC article. Review.
References
-
- Pociot F, Lernmark Å. Genetic risk factors for type 1 diabetes. Lancet. 2016;387(10035):2331–9. - PubMed
-
- Hörber S, Achenbach P, Schleicher E, Peter A. Harmonization of immunoassays for biomarkers in diabetes mellitus. Biotechnol Adv. 2020;39:107359. - PubMed
-
- Undlien DE, Kockum I, Rønningen KS, Lowe R, Saanjeevi CB, Graham J, et al. HLA associations in type 1 diabetes among patients not carrying high-risk DR3-DQ2 or DR4-DQ8 haplotypes. Tissue Antigens. 1999;54(6):543–51. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

