Vacuole and mitochondria patch protein Mcp1 of Saccharomyces boulardii impairs the oxidative stress response of Candida albicans by regulating 2-phenylethanol
- PMID: 40259315
- PMCID: PMC12013155
- DOI: 10.1186/s12934-025-02721-0
Vacuole and mitochondria patch protein Mcp1 of Saccharomyces boulardii impairs the oxidative stress response of Candida albicans by regulating 2-phenylethanol
Abstract
Background: Vacuole and mitochondria patch (vCLAMP) protein Mcp1 is crucial in eukaryotic cells response to environmental stress, but the mechanism of Mcp1 in Saccharomyces boulardii (S. boulardii) against pathogenic fungi is unclear.
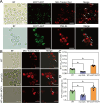
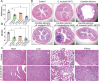
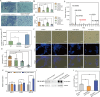
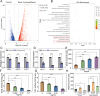
Results: This work first explored the role of Mcp1 in S. boulardii against Candida albicans (C. albicans). The results showed that Mcp1 located on the vacuolar and mitochondrial membrane of S. boulardii. Overexpression of Mcp1 inhibited the adhesion and hyphal formation of C. albicans in vitro. The mice model of intestinal infection revealed that WT-pGK1-MCP1 mutant enhanced the ability of S. boulardii antagonize C. albicans infecting gut. High performance liquid chromatography-mass spectrometry analysis demonstrated that overexpressing Mcp1 promoted the production of 2-phenylethanol. The latter is a secondary metabolite of S. boulardii, and can inhibit the adhesion and biofilm formation of C. albicans. The reverse transcription polymerase chain reaction and western blotting results confirmed Mcp1 promoted the production of 2-phenylethanol by regulating the expression level of Aro10. Notably, RNA-sequencing and Gene Ontology enrichment analyses showed that 2-phenylethanol impaired the oxidative stress response of C. albicans.
Conclusion: This work reveals the critical role of Mcp1 in S. boulardii against C. albicans by regulating 2-phenylethanol metabolism, which provide a theoretical basis for S. boulardii as antifungal biologic therapy to prevent and treat of Candida infection.
Keywords: 2-phenylethanol; Antagonism; Mcp1; Organelle membranes; Oxidative stress response.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Ethics Committee of Shandong First Medical University & Shandong Academy of Medical Sciences (permit number W202302270104). Informed consent: Informed consent was obtained from all individual participants included in the study. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Antifungal bio-coating of endotracheal tube built by overexpressing the MCP1 gene of Saccharomyces boulardii and employing hydrogel as a "house" to antagonize Candida albicans.Biomater Res. 2023 Oct 5;27(1):97. doi: 10.1186/s40824-023-00443-1. Biomater Res. 2023. PMID: 37798667 Free PMC article.
-
Capric acid secreted by S. boulardii inhibits C. albicans filamentous growth, adhesion and biofilm formation.PLoS One. 2010 Aug 10;5(8):e12050. doi: 10.1371/journal.pone.0012050. PLoS One. 2010. PMID: 20706577 Free PMC article.
-
Saccharomyces boulardii decreases inflammation and intestinal colonization by Candida albicans in a mouse model of chemically-induced colitis.Med Mycol. 2007 Dec;45(8):691-700. doi: 10.1080/13693780701523013. Med Mycol. 2007. PMID: 17885943
-
The antagonistic effect of Saccharomyces boulardii on Candida albicans filamentation, adhesion and biofilm formation.FEMS Yeast Res. 2009 Dec;9(8):1312-21. doi: 10.1111/j.1567-1364.2009.00559.x. Epub 2009 Aug 5. FEMS Yeast Res. 2009. PMID: 19732158
-
Involvement of amyloid proteins in the formation of biofilms in the pathogenic yeast Candida albicans.Res Microbiol. 2021 Apr-May;172(3):103813. doi: 10.1016/j.resmic.2021.103813. Epub 2021 Jan 28. Res Microbiol. 2021. PMID: 33515679 Review.
References
-
- Ghugari R, Tsao S, Schmidt M, Bonneil É, Brenner C, Verreault A. Mechanisms to reduce the cytotoxicity of Pharmacological nicotinamide concentrations in the pathogenic fungus Candida albicans. FEBS J. 2021;288:3478–506. - PubMed
-
- Sasaki H, Kurakado S, Matsumoto Y, Yoshino Y, Sugita T, Koyama K, Kinoshita K. Enniatins from a marine-derived fungus Fusarium sp. inhibit biofilm formation by the pathogenic fungus Candida albicans. J Nat Med. 2023;77:455–63. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

