Associations of blood RNA biomarkers and circulating tumour cells in patients with previously untreated metastatic colorectal cancer
- PMID: 40259317
- PMCID: PMC12013160
- DOI: 10.1186/s12885-025-14098-9
Associations of blood RNA biomarkers and circulating tumour cells in patients with previously untreated metastatic colorectal cancer
Abstract
Background: In patients with metastatic colorectal cancer, analysis of the number of basal circulating tumour cells (bCTCs) has been shown to be a strong prognostic indicator. In this study, we aim to explore the potential associations between whole blood mRNA and microRNA expression profiles and bCTC counts, tumour mutations and prognosis in untreated metastatic colorectal cancer patients.
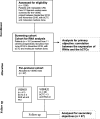
Methods: A total of 151 patients previously screened for inclusion in two clinical trials (VISNÚ1 and VISNÚ2) were enrolled in this study. Real-time quantitative PCR (qPCR) analyses were performed to determine the whole blood expression of selected RNAs (mRNAs and microRNAs) involved in the metastatic process. The CellSearch system was used to enumerate circulating tumour cells. The primary objective was to correlate RNA expression with the number of bCTCs, while the secondary objectives were to investigate the relationship between the levels of circulating RNA biomarkers in whole blood and the clinical, pathological, and molecular characteristics and prognosis of patients with metastatic colorectal cancer.
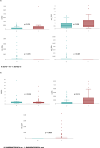
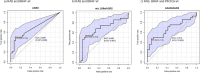
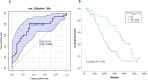
Results: bCTC count was significantly associated with AGR2 mRNA in the entire cohort of 151 patients. AGR2, ADAR1 and LGR5 were associated with the number of bCTC, both in the subgroup with bCTC ≥ 3 and in the subgroup with native RAS/BRAF/PIK3 CA tumours. In patients with RAS/BRAF/PIK3 CA mutations no correlations with bCTC were detected, but an upregulation of miR-224-5p and the stemness marker LGR5 and a downregulation of immune regulatory CD274 were found. Lower levels of miR-106a-5p/miR-26a-5p were associated with shorter overall survival, with independent statistical significance in the multivariate analysis.
Conclusions: A correlation was identified between the levels of a subset of whole blood RNAs, including AGR2, ADAR1, and LGR5, and the number of bCTC and RAS/BRAF/PIK3 CA mutational status. Furthermore, another set of whole blood RNAs, specifically miR-106a-5p and miR-26a-5p, was found to be associated with poor prognosis. This may be helpful for risk stratification.
Trial registration: Clinical Trials Gov. NCT01640405 and NCT01640444. Registered on 13 June 2012. https://clinicaltrials.gov/ .
Keywords: Circulating tumour cells; Liquid biopsy; Metastatic colorectal cancer; Prognosis; RNA biomarkers.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted in accordance with Good Clinical Practice guidelines, and the Declaration of Helsinki and its subsequent amendments. The study was approved by the Research Ethics Committee CEIC Hospital Clínico San Carlos (Madrid), which served as the Institutional Review Board for all participating centres. The study protocol was also approved by the Spanish Agency of Medicines and Medical Devices. Consent for publication: Not required. Competing interests: MV-A has received grants and personal fees from Roche, personal fees from Merck, Amgen, Sanofi, Servier, Celgene, MSD and Bayer and honoraria for speaker/consulting roles from Amgen, Bayer, Merck, MSD, Roche, Sanofi and Servier. BGS has received travel grants from Amgen, Astra Zeneca, Merck, and Servier, and grants from Amgen, Sanofi, Gilead, Celgene, Bristol Myers Squibb, and Amgen. JS has done speaking honoraria for Ipsen, Lilly, Merck, MSD, Pfizer, Roche, Shire, and Servier, advisory roles for Amgen, Bayer, Bristol-Myers Squibb, Celgene, Ipsen, Merck, Roche, Sanofi, and Servier, and received travelling and accommodation support from Ipsen and Merck. FR has received payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events fromRoche, Merck-Serono, AMGEN, Sanofi-Aventis, Servier, MSD, and Support for attending meetings and/or travel from Roche, MSD, and Servier. PG-A has received payment as speaker’ bureau for Amgen, Roche, Merck-Serono, MSD, Sanofi-Aventis, Pierre Fabre and Servier and received travel, accommodations and expenses from Amgen, Roche, Merck-Serono, Sanofi-Aventis, MSD, Pierre Fabre and Servier and payments from advisory activities from Amgen, Roche, Merck-Serono, MSD, Sanofi-Aventis, Pierre Fabre and Servier. RLL has stock and other ownership interests in MTrap and Nasasbiotech SL, and received consulting or advisory payments for Bayer, Bristol-Myers Squibb, Janssen, Lilly, MSD, Roche, and Novartis, received institutional research funding from Lilly, Merck, and Roche, and r travel, accommodation, and expenses from Pierre Fabre, Roche and Tesaro. CG-P has received travel, accommodation, and expenses from Sanofi/Aventis. ED-R has done consulting or advisory roles for Amgen, Bayer, Genomica, Merck Serono and Servier, speakers’ bureaus for MSD and Servier, and received research funding from Amgen, AstraZeneca, Merck Serono, Roche and Sysmex. EA has received honoraria for advisory role from Amgen, Bayer, Sanofi, Incyte and Pierre Fabre. MT-F, PJ-F, GP-C, SG, AS, MS, AR-A, JMV declare no potential conflicts of interest.
Figures
Similar articles
-
Association Between Baseline Circulating Tumor Cells, Molecular Tumor Profiling, and Clinical Characteristics in a Large Cohort of Chemo-naïve Metastatic Colorectal Cancer Patients Prospectively Collected.Clin Colorectal Cancer. 2020 Sep;19(3):e110-e116. doi: 10.1016/j.clcc.2020.02.014. Epub 2020 Mar 6. Clin Colorectal Cancer. 2020. PMID: 32278676 Clinical Trial.
-
Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: a retrospective, exploratory analysis of the CORRECT trial.Lancet Oncol. 2015 Aug;16(8):937-48. doi: 10.1016/S1470-2045(15)00138-2. Epub 2015 Jul 13. Lancet Oncol. 2015. PMID: 26184520 Free PMC article.
-
Association of Circulating Tumor Cells and Tumor Molecular Profile With Clinical Outcomes in Patients With Previously Untreated Metastatic Colorectal Cancer: A Pooled Analysis of the Phase III VISNÚ-1 and Phase II VISNÚ-2 Randomized Trials.Clin Colorectal Cancer. 2023 Jun;22(2):222-230. doi: 10.1016/j.clcc.2023.02.004. Epub 2023 Feb 21. Clin Colorectal Cancer. 2023. PMID: 36944559
-
Prognostic and predictive biomarkers for anti-EGFR monoclonal antibody therapy in RAS wild-type metastatic colorectal cancer: a systematic review and meta-analysis.BMC Cancer. 2023 Nov 16;23(1):1117. doi: 10.1186/s12885-023-11600-z. BMC Cancer. 2023. PMID: 37974093 Free PMC article.
-
The use of circulating microRNAs as diagnostic biomarkers in colorectal cancer.Cancer Biomark. 2015;15(2):103-13. doi: 10.3233/CBM-140456. Cancer Biomark. 2015. PMID: 25547322 Review.
References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. 10.3322/caac.21834. Epub 2024 Apr 4. PMID: 38572751. - PubMed
-
- Ciardiello F, Ciardiello D, Martini G, Napolitano S, Tabernero J, Cervantes A. Clinical management of metastatic colorectal cancer in the era of precision medicine. CA Cancer J Clin. 2022;72:372–401. 10.3322/caac.21728. - PubMed
-
- Heitzer E, Haque IS, Roberts CES, Speicher MR. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet. 2019;20:71–88. 10.1038/s41576-018-0071-5. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

