Engineering a PhrC-RapC-SinR quorum sensing molecular switch for dynamic fine-tuning of menaquinone-7 synthesis in Bacillus subtilis
- PMID: 40259323
- PMCID: PMC12010548
- DOI: 10.1186/s12934-025-02714-z
Engineering a PhrC-RapC-SinR quorum sensing molecular switch for dynamic fine-tuning of menaquinone-7 synthesis in Bacillus subtilis
Abstract
Background: Menaquinone-7 (MK-7) is a valuable vitamin K2 produced by Bacillus subtilis. Although many strategies have been adopted to increase the yield of MK-7 in B. subtilis, the effectiveness of these common approaches is not high because long metabolic synthesis pathways and numerous bypass pathways competing for precursors with MK-7 synthesis. Regarding the modification of bypass pathways, studies of common static metabolic engineering method such as knocking out genes involved in side pathway have been reported previously. Since byproductsphenylalanine(Phe), tyrosine (Tyr), tryptophan (Trp), folic acid, dihydroxybenzoate, hydroxybutanone in the MK-7 synthesis pathway are indispensable for cell growth, the complete knockout of the bypass pathway restricts cell growth, resulting in limited increase in MK-7 synthesis. Dynamic regulation via quorum sensing (QS) provides a cost-effective strategy to harmonize cell growth and product synthesis, eliminating the need for pricey inducers. SinR, a transcriptional repressor, is crucial in suppressing biofilm formation, a process closely intertwined with MK-7 biosynthesis. Given this link, we targeted SinR to construct a dynamic regulatory system, aiming to modulate MK-7 production by leveraging SinR's regulatory influence.
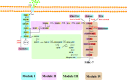
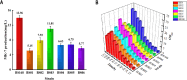
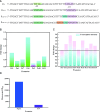
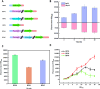
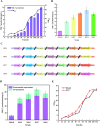
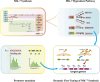
Results: A modular PhrC-RapC-SinR QS system is developed to dynamic regulate side pathway of MK-7. In this study, first, we analyzed the SinR-based gene expression regulation system in B. subtilis 168 (BS168). We constructed a promoter library of different abilities, selected suitable promoters from the library, and performed mutation screening on the selected promoters. Furthermore, we constructed a PhrC-RapC-SinR QS system to dynamically control the synthesis of Phe, Tyr, Trp, folic acid, dihydroxybenzoate, hydroxybutanone in MK-7 synthesis in BS168. Cell growth and efficient synthesis of the MK-7 production can be dynamically balanced by this QS system. Using this system to balance cell growth and product fermentation, the MK-7 yield was ultimately increased by 6.27-fold, from 13.95 mg/L to 87.52 mg/L.
Conclusion: In summary, the PhrC-RapC-SinR QS system has been successfully integrated with biocatalytic functions to achieve dynamic metabolic pathway control in BS168, which has potential applicability to a large number of microorganisms to fine-tune gene expression and enhance the production of metabolites.
Keywords: Bacillus subtilis; MK-7; PhrC-RapC; SinR.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This article does not contain studies with human participants or animals performed by any of the authors. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Site-directed mutagenesis of the quorum-sensing transcriptional regulator SinR affects the biosynthesis of menaquinone in Bacillus subtilis.Microb Cell Fact. 2021 Jun 7;20(1):113. doi: 10.1186/s12934-021-01603-5. Microb Cell Fact. 2021. PMID: 34098969 Free PMC article.
-
Engineering a ComA Quorum-Sensing circuit to dynamically control the production of Menaquinone-4 in Bacillus subtilis.Enzyme Microb Technol. 2021 Jun;147:109782. doi: 10.1016/j.enzmictec.2021.109782. Epub 2021 Mar 18. Enzyme Microb Technol. 2021. PMID: 33992404
-
Engineering a Bifunctional Phr60-Rap60-Spo0A Quorum-Sensing Molecular Switch for Dynamic Fine-Tuning of Menaquinone-7 Synthesis in Bacillus subtilis.ACS Synth Biol. 2019 Aug 16;8(8):1826-1837. doi: 10.1021/acssynbio.9b00140. Epub 2019 Jul 1. ACS Synth Biol. 2019. PMID: 31257862
-
New aspects of microbial vitamin K2 production by expanding the product spectrum.Microb Cell Fact. 2021 Apr 13;20(1):84. doi: 10.1186/s12934-021-01574-7. Microb Cell Fact. 2021. PMID: 33849534 Free PMC article. Review.
-
Biofilm reactors as a promising method for vitamin K (menaquinone-7) production.Appl Microbiol Biotechnol. 2019 Jul;103(14):5583-5592. doi: 10.1007/s00253-019-09913-w. Epub 2019 Jun 1. Appl Microbiol Biotechnol. 2019. PMID: 31152205 Review.
Cited by
-
A quorum sensing-controlled type I CRISPRi toolkit for dynamically regulating metabolic flux.Nucleic Acids Res. 2025 Jul 19;53(14):gkaf693. doi: 10.1093/nar/gkaf693. Nucleic Acids Res. 2025. PMID: 40682826 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

