PTEN as a prognostic factor for radiotherapy plus immunotherapy response in nasopharyngeal carcinoma
- PMID: 40259377
- PMCID: PMC12010603
- DOI: 10.1186/s12951-025-03315-z
PTEN as a prognostic factor for radiotherapy plus immunotherapy response in nasopharyngeal carcinoma
Abstract
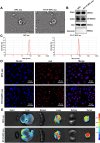
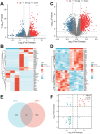
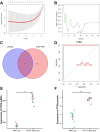
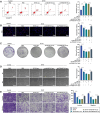
Background: In the context of nasopharyngeal carcinoma (NPC) treatment, radiotherapy combined with immunotherapy (IR + RT) is gaining traction. This study focuses on analyzing exosomal proteins, particularly Phosphatase and Tensin Homolog (PTEN), for predicting the efficacy of NPC treatments. Serum samples from NPC patients and IR + RT recipients were utilized for exosome (Exo) extraction and subsequent transcriptomic and proteomic analyses to identify treatment-related proteins. Flow cytometry of cells and exosomal analysis were performed to examine these proteins. In vitro experiments using C666-1 cells and their Exos explored various cellular responses, while a murine subcutaneous NPC model investigated the impact of PTEN modulation on tumor growth and the immune microenvironment.
Results: The study demonstrated that PTEN serves as a crucial predictive biomarker, with its expression changes correlated with M2 macrophage polarization and CD8+ T cell activity. This highlights the potential significance of PTEN in predicting treatment outcomes and influencing the immune response in NPC.
Conclusion: The findings suggest that PTEN could play a key role in enhancing the efficacy of NPC radiotherapy and immunotherapy. By shedding light on PTEN's impact on tumor growth and the immune microenvironment, this study lays the groundwork for future personalized therapeutic strategies in NPC treatment.
Keywords: Exosome; Immunotherapy; Machine learning; Nanofluidic technology; Nasopharyngeal carcinoma; Phosphatase and Tensin Homolog; Radiotherapy; Tumor microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Written informed consent was obtained from all participants, and the study was approved by Scientific Research Ethics Committee of China Medical University. All mouse experiments were conducted in accordance with institutional guidelines and approved by the Animal Care and Use Committee of China Medical University. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
KMO-driven metabolic reconfiguration and its impact on immune cell infiltration in nasopharyngeal carcinoma: a new avenue for immunotherapy.Cancer Immunol Immunother. 2025 Feb 1;74(3):75. doi: 10.1007/s00262-024-03928-7. Cancer Immunol Immunother. 2025. PMID: 39891699 Free PMC article.
-
Tumor cell-derived exosome RNF126 affects the immune microenvironment and promotes nasopharyngeal carcinoma progression by regulating PTEN ubiquitination.Apoptosis. 2022 Aug;27(7-8):590-605. doi: 10.1007/s10495-022-01738-9. Epub 2022 Jun 19. Apoptosis. 2022. PMID: 35717659
-
Exosomes derived from γδ-T cells synergize with radiotherapy and preserve antitumor activities against nasopharyngeal carcinoma in immunosuppressive microenvironment.J Immunother Cancer. 2022 Feb;10(2):e003832. doi: 10.1136/jitc-2021-003832. J Immunother Cancer. 2022. PMID: 35105688 Free PMC article.
-
The Role of PTEN in Nasopharyngeal Carcinoma.Front Biosci (Landmark Ed). 2024 May 10;29(5):179. doi: 10.31083/j.fbl2905179. Front Biosci (Landmark Ed). 2024. PMID: 38812313 Review.
-
Combinatorial strategies of radiotherapy and immunotherapy in nasopharyngeal carcinoma.Chin Clin Oncol. 2018 Apr;7(2):15. doi: 10.21037/cco.2018.04.05. Chin Clin Oncol. 2018. PMID: 29764160 Review.
References
-
- Wong KCW, Hui EP, Lo K-W, Lam WKJ, Johnson D, Li L, et al. Nasopharyngeal carcinoma: an evolving paradigm. Nat Rev Clin Oncol. 2021. 10.1038/s41571-021-00524-x. - PubMed
-
- King AD. MR imaging of nasopharyngeal carcinoma. Magn Reson Imaging Clin N Am. 2022. 10.1016/j.mric.2021.06.015. - PubMed
-
- Morisaki T, Ohguri T, Yahara K, Nakahara S, Kakinouchi S, Itamura H, et al. Salvage re-irradiation with intensity-modulated radiotherapy, chemotherapy combined with hyperthermia for local recurrence of nasopharyngeal carcinoma after chemoradiotherapy. J UOEH. 2021. 10.7888/juoeh.43.355. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

