Felodipine Promotes the Recovery of Mice With Spinal Cord Injury by Activating Macrolipophagy Through the AMPK-mTOR Pathway
- PMID: 40259510
- PMCID: PMC12011640
- DOI: 10.1111/jcmm.70543
Felodipine Promotes the Recovery of Mice With Spinal Cord Injury by Activating Macrolipophagy Through the AMPK-mTOR Pathway
Abstract
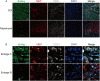
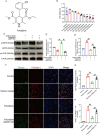
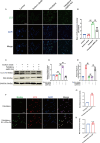
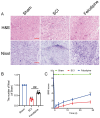
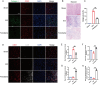
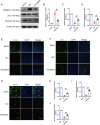
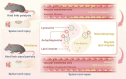
Spinal cord injury (SCI) is a serious clinical condition characterised by extensive mechanical damage that compromises the tissue structure and microenvironment of the affected area. This damage leads to the formation of fibrotic blood vessels and impaired energy metabolism, both of which hinder recovery. Felodipine, a clinically approved antihypertensive drug, acts as a selective calcium antagonist, primarily inhibiting extracellular calcium influx in arteriolar smooth muscle and selectively dilating arterioles. Additionally, felodipine has been demonstrated to induce autophagy. Considering these properties collectively, we hypothesised that felodipine could modulate the microenvironment of the injured spinal cord. In this study, we employed immunofluorescence and Western blot analyses to evaluate the effects of felodipine on microenvironment repair and neuroprotection, both in vitro and in vivo. Particular attention was given to its regulatory role in AMPK-mTOR pathway-mediated macrolipophagy. Our results demonstrated that felodipine effectively improved the injured spinal cord microenvironment by activating macrolipophagy, facilitating the clearance of myelin debris. Furthermore, felodipine promoted the restoration of endothelial cell tight junctions, thereby enhancing the integrity of the blood-spinal cord barrier. This attenuation of barrier disruption after SCI contributed to improved neuronal survival. These findings expanded the clinical application prospect of felodipine and presented new therapeutic avenues for treating SCI.
Keywords: AMPK; Felodipine; macrolipophagy; microenvironment; spinal cord injury.
© 2025 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Wnt-3a improves functional recovery through autophagy activation via inhibiting the mTOR signaling pathway after spinal cord injury.Neurosci Lett. 2020 Oct 15;737:135305. doi: 10.1016/j.neulet.2020.135305. Epub 2020 Aug 17. Neurosci Lett. 2020. PMID: 32818590
-
Scopoletin Activates Adenosine Monophosphate-Activated Protein Kinase/Mammalian Target of Rapamycin Signaling Pathway and Improves Functional Recovery after Spinal Cord Injury in Rats.Pharmacology. 2020;105(5-6):349-359. doi: 10.1159/000503866. Epub 2020 Jan 17. Pharmacology. 2020. Retraction in: Pharmacology. 2024;109(1):65. doi: 10.1159/000534434. PMID: 31955175 Retracted.
-
Resveratrol improves neurological outcome and neuroinflammation following spinal cord injury through enhancing autophagy involving the AMPK/mTOR pathway.Mol Med Rep. 2018 Aug;18(2):2237-2244. doi: 10.3892/mmr.2018.9194. Epub 2018 Jun 19. Mol Med Rep. 2018. PMID: 29956767
-
Valproic Acid: A Potential Therapeutic for Spinal Cord Injury.Cell Mol Neurobiol. 2021 Oct;41(7):1441-1452. doi: 10.1007/s10571-020-00929-9. Epub 2020 Jul 28. Cell Mol Neurobiol. 2021. PMID: 32725456 Free PMC article. Review.
-
Beneficial Effects of Resveratrol-Mediated Inhibition of the mTOR Pathway in Spinal Cord Injury.Neural Plast. 2018 Mar 26;2018:7513748. doi: 10.1155/2018/7513748. eCollection 2018. Neural Plast. 2018. PMID: 29780409 Free PMC article. Review.
References
-
- Mautes A. E., Weinzierl M. R., Donovan F., and Noble L. J., “Vascular Events After Spinal Cord Injury: Contribution to Secondary Pathogenesis,” Physical Therapy 80, no. 7 (2000): 673–687. - PubMed
-
- Martirosyan N. L., Feuerstein J. S., Theodore N., Cavalcanti D. D., Spetzler R. F., and Preul M. C., “Blood Supply and Vascular Reactivity of the Spinal Cord Under Normal and Pathological Conditions,” Journal of Neurosurgery. Spine 15, no. 3 (2011): 238–251, 10.3171/2011.4.SPINE10543. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

