Phenoconversion of CYP3A4, CYP2C19 and CYP2D6 in Pediatrics, Adolescents and Young Adults With Lymphoma: Rationale and Design of the PEGASUS Study
- PMID: 40259519
- PMCID: PMC12011636
- DOI: 10.1111/cts.70209
Phenoconversion of CYP3A4, CYP2C19 and CYP2D6 in Pediatrics, Adolescents and Young Adults With Lymphoma: Rationale and Design of the PEGASUS Study
Abstract
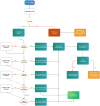
Phenoconversion is the discrepancy between genotype-predicted phenotype and clinical phenotype, due to nongenetic factors. In oncology patients, the impact of phenoconversion on drug selection, efficacy, toxicity, and treatment outcomes is unknown. This study will assess acceptability and feasibility of investigating phenoconversion using probe medications in a pediatric and adolescent and young adult (AYA) oncology population. This prospective, single-arm, single-blind, nonrandomized feasibility study, will enroll individuals aged 6-25 years with a new diagnosis of Hodgkin Lymphoma or Non-Hodgkin Lymphoma. Genotyping will be performed at baseline using whole genome sequencing or targeted panel testing. Longitudinal phenotyping will be conducted throughout the cancer treatment using exogenous oral enzyme-specific probes, specifically subtherapeutic dextromethorphan (CYP2D6) and omeprazole (CYP2C19, CYP3A4) for enzyme activity assessment. The primary outcome measure will be the proportion of patients who consent to the study and successfully complete baseline and at least two longitudinal time points with valid probe drug metabolic ratio measurements. Secondary outcomes include classification of clinical phenotypes based on probe drug metabolic ratios, probe drug safety, barriers to consent, acceptability of pharmacogenomic and phenoconversion testing, longitudinal genotype/phenotype concordance, inflammatory profiles, and patient and disease factors influencing phenoconversion. The trial has received ethics approval (2023/ETH1954) and is registered at ClinicalTrials.gov (NCT06383338). Findings will be disseminated through peer-reviewed publications and professional conferences, providing critical insights to advance the understanding of phenoconversion in oncology from pediatrics to adults. These results will help shape future research and drive the implementation of more personalized precision medicine strategies for all people with cancer.
Keywords: Australia; Phenoconversion; oncology; pharmacogenomic implementation; pharmacogenomics.
© 2025 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
An analysis of allele, genotype and phenotype frequencies, actionable pharmacogenomic (PGx) variants and phenoconversion in 5408 Australian patients genotyped for CYP2D6, CYP2C19, CYP2C9 and VKORC1 genes.J Neural Transm (Vienna). 2019 Jan;126(1):5-18. doi: 10.1007/s00702-018-1922-0. Epub 2018 Sep 6. J Neural Transm (Vienna). 2019. PMID: 30191366
-
Evaluation of Mutual Drug-Drug Interaction within Geneva Cocktail for Cytochrome P450 Phenotyping using Innovative Dried Blood Sampling Method.Basic Clin Pharmacol Toxicol. 2016 Sep;119(3):284-90. doi: 10.1111/bcpt.12586. Epub 2016 Apr 25. Basic Clin Pharmacol Toxicol. 2016. PMID: 27009433 Clinical Trial.
-
Combined phenotypic assessment of CYP1A2, CYP2C19, CYP2D6, CYP3A, N-acetyltransferase-2, and xanthine oxidase with the "Cooperstown cocktail".Clin Pharmacol Ther. 2000 Oct;68(4):375-83. doi: 10.1067/mcp.2000.109519. Clin Pharmacol Ther. 2000. PMID: 11061577
-
CYP2D6 pharmacogenetics and phenoconversion in personalized medicine.Expert Opin Drug Metab Toxicol. 2022 Nov;18(11):769-785. doi: 10.1080/17425255.2022.2160317. Epub 2023 Jan 3. Expert Opin Drug Metab Toxicol. 2022. PMID: 36597259 Free PMC article. Review.
-
Addressing phenoconversion: the Achilles' heel of personalized medicine.Br J Clin Pharmacol. 2015 Feb;79(2):222-40. doi: 10.1111/bcp.12441. Br J Clin Pharmacol. 2015. PMID: 24913012 Free PMC article. Review.
References
-
- Foulds J. A., Maggo S. D., and Kennedy M. A., “Personalised Prescribing in Psychiatry: Has Pharmacogenomics Delivered on Its Promise?,” Australian and New Zealand Journal of Psychiatry 50, no. 6 (2016): 509–510. - PubMed
-
- Zhou S. F., Xue C. C., Yu X. Q., Li C., and Wang G., “Clinically Important Drug Interactions Potentially Involving Mechanism‐Based Inhibition of Cytochrome P450 3A4 and the Role of Therapeutic Drug Monitoring,” Therapeutic Drug Monitoring 29, no. 6 (2007): 687–710. - PubMed
-
- Shah R. R. and Smith R. L., “Inflammation‐Induced Phenoconversion of Polymorphic Drug Metabolizing Enzymes: Hypothesis With Implications for Personalized Medicine,” Drug Metabolism and Disposition 43, no. 3 (2015): 400–410. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

