Genome Wide Association Study (GWAS) Identifies Novel Genetic Loci for Second-Generation Antipsychotics (SGA)-Induced Metabolic Syndrome (MetS)
- PMID: 40259522
- PMCID: PMC12011641
- DOI: 10.1111/cts.70216
Genome Wide Association Study (GWAS) Identifies Novel Genetic Loci for Second-Generation Antipsychotics (SGA)-Induced Metabolic Syndrome (MetS)
Abstract
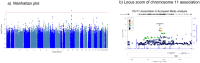
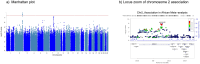
Second-generation antipsychotics (SGA) are widely used for treating psychiatric disorders; however, their use is associated with an increased risk of metabolic syndrome (MetS). To identify common genetic associations of SGA-induced metabolic syndrome (SGA-MetS), we conducted a genome-wide association study (GWAS) in a diverse patient population within the BioVU and BioMe electronic health records (EHRs)-linked biobanks. Additionally, we performed Mendelian Randomization (MR) analysis to investigate the association between the individual metabolic parameters comprising MetS (body mass index [BMI], fasting glucose, blood pressure, HDL, and triglycerides) and SGA-MetS. The meta-analysis of European ancestry GWAS from BioVU and BioMe (N = 9248) identified a genome-wide signal (rs61900075, β = -0.27, SE = 0.05, p = 1.6 × 10-8) on chromosome 11. Multiple associated variants met the suggestive level of association (p ≤ 10-5) in the PELO-ITGA1 locus on chromosome 5 and were associated among the Hispanic Ancestry within BioMe. The meta-analysis of the African Ancestry patients of BioVU and BioMe (N = 2018) identified multiple genome-wide signals that were functionally mapped to NPPC-DIS3L2 in chromosome 2. Finally, the inverse-variance weighted average MR (BMI: OR = 1.2, 95% CI: 1.1-1.4, p = 0.002) showed that genetically predicted, higher BMI was associated with an increased risk of SGA-MetS. Similar results were seen in the sensitivity analyses using the weighted median and Egger MR. This study identified novel variants for SGA-MetS and suggested a role of BMI in increasing the risk of SGA-MetS. The findings highlight the value of EHR biobanks for identifying the genetics underlying SGA-MetS. The associations in chromosome 2 and 5 will need further replication.
© 2025 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Carton L., Cottencin O., Lapeyre‐Mestre M., et al., “Off‐Label Prescribing of Antipsychotics in Adults, Children and Elderly Individuals: A Systematic Review of Recent Prescription Trends,” Current Pharmaceutical Design 21, no. 23 (2015): 3280–3297. - PubMed
-
- De Hert M., Detraux J., van Winkel R., Yu W., and Correll C. U., “Metabolic and Cardiovascular Adverse Effects Associated With Antipsychotic Drugs,” Nature Reviews. Endocrinology 8, no. 2 (2011): 114–126. - PubMed
-
- Berenson G. S., Srinivasan S. R., Bao W., Newman W. P., Tracy R. E., and Wattigney W. A., “Association Between Multiple Cardiovascular Risk Factors and Atherosclerosis in Children and Young Adults. The Bogalusa Heart Study,” New England Journal of Medicine 338, no. 23 (1998): 1650–1656. - PubMed
-
- Tu T. H., Huang K. L., Bai Y. M., et al., “Exposure to Second‐Generation Antipsychotics and Risk of Type 2 Diabetes Mellitus in Adolescents and Young Adults: A Nationwide Study in Taiwan,” Journal of Clinical Psychiatry 80, no. 2 (2019): 18m12284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

