Desensitization With Proteasome Inhibition and Costimulation Blockade Modulates the Xenoreactive Humoral Response in Nonhuman Primate Xenotransplantation
- PMID: 40259710
- PMCID: PMC12012413
- DOI: 10.1111/xen.70045
Desensitization With Proteasome Inhibition and Costimulation Blockade Modulates the Xenoreactive Humoral Response in Nonhuman Primate Xenotransplantation
Abstract
Introduction: "Delayed" antibody-mediated xenograft rejection is one of the most important obstacles to clinical application of pig organ xenografts. The aim of this study was to assess the impact of a structured desensitization regimen including proteasome inhibition and next-generation costimulation blockade on xenoreactive antibodies.
Methods: Rhesus macaques with moderate-high pre-treatment xenoreactive antibody titers (N = 2) were selected. Recipients received twice-weekly carfilzomib (20 mg/m2), anti-CD154 (20 mg/kg) every other week, and CD4 and CD20 lymphocyte cell depletion. Bone marrow was acquired to assess plasma cell depletion in response to proteasome inhibition. A flow cytometry-based xenoreactive crossmatch assay was performed to assess levels of circulating xenoreactive antibodies.
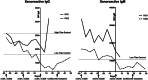
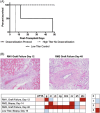
Results: The desensitization regimen resulted in a >50% depletion of CD38+CD27+ bone marrow plasma cells; these changes were progressive over the duration of the desensitization treatment period. The desensitization strategy and plasma cell depletion resulted in a progressive reduction in anti-pig IgG antibodies. Following xenotransplantation, both desensitized recipients demonstrated superior graft survival to a highly xenoreactive recipient (MST 30 days vs. 6 days), but neither desensitized recipient experienced prolonged graft survival.
Conclusions: A structured desensitization regimen including proteasome inhibition and costimulation blockade results in plasma cell depletion and resultant reduction in circulating xenoreactive anti-pig IgG antibodies, with a modest improvement in xenograft survival. This desensitization regimen has promise for pig-to-NHP xenotransplant models.
Keywords: antibody; proteasome inhibition; xenotransplantation.
© 2025 The Author(s). Xenotransplantation published by Wiley Periodicals LLC.
Figures


Similar articles
-
Pretransplant Desensitization with Costimulation Blockade and Proteasome Inhibitor Reduces DSA and Delays Antibody-Mediated Rejection in Highly Sensitized Nonhuman Primate Kidney Transplant Recipients.J Am Soc Nephrol. 2019 Dec;30(12):2399-2411. doi: 10.1681/ASN.2019030304. Epub 2019 Oct 28. J Am Soc Nephrol. 2019. PMID: 31658991 Free PMC article.
-
Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model.Xenotransplantation. 2015 May-Jun;22(3):221-30. doi: 10.1111/xen.12166. Epub 2015 Apr 3. Xenotransplantation. 2015. PMID: 25847130 Free PMC article.
-
An IgM Cleaving Enzyme for Clearance of Anti-Pig Xenoreactive Antibodies in a Nonhuman Primate Model.Xenotransplantation. 2025 Jan-Feb;32(1):e70023. doi: 10.1111/xen.70023. Xenotransplantation. 2025. Retraction in: Xenotransplantation. 2025 Jul-Aug;32(4):e70070. doi: 10.1111/xen.70070. PMID: 39960355 Retracted.
-
Survival following orthotopic cardiac xenotransplantation between juvenile baboon recipients and concordant and discordant donor species: foundation for clinical trials.World J Surg. 1997 Nov-Dec;21(9):943-50. doi: 10.1007/s002689900331. World J Surg. 1997. PMID: 9361509 Review.
-
Preventing T cell rejection of pig xenografts.Int J Surg. 2015 Nov;23(Pt B):285-290. doi: 10.1016/j.ijsu.2015.07.722. Epub 2015 Aug 22. Int J Surg. 2015. PMID: 26306770 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

