Retinal Oxygen Kinetics and Hemodynamics in Choroidal Melanoma After Iodine-125 Plaque Radiotherapy Using a Novel Structural-Functional Imaging Analysis System
- PMID: 40259788
- PMCID: PMC12012311
- DOI: 10.1002/cam4.70854
Retinal Oxygen Kinetics and Hemodynamics in Choroidal Melanoma After Iodine-125 Plaque Radiotherapy Using a Novel Structural-Functional Imaging Analysis System
Abstract
Background: To investigate the changes in retinal oxygen kinetics and hemodynamics in patients with choroidal melanoma (CM) within 2 years before and after iodine-125 plaque radiotherapy (PRT) using a novel noninvasive structure-functional imaging analysis system.
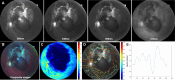
Methods: A novel noninvasive cost-effective imaging analysis system that integrates multimodal structural and functional retinal imaging techniques has been used, which allows rapid acquisition of vascular structural, hemodynamic, and oxygenation metrics using multispectral imaging (MSI) and laser speckle contrast imaging (LSCI) techniques. Follow-ups have been arranged at the time before plaque implantation surgery, and 1 month, 3 months, 6 months, 12 months, 18 months, and 24 months after iodine-125 plaque removal.
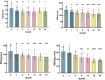
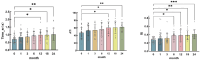
Results: CM patients after PRT demonstrated a significant decrease in retinal arterial oxygen concentration (CO2 a), arterial oxygen saturation (SO2 a), oxygen utilization (SO2 av, CO2 av), and metabolism (oxygen extraction fraction, OEF) over time. However, there was no significant difference in SO2 and CO2 compared with healthy controls. Systolic time (Time_sr), acceleration time index (ATI), and resistivity index (RI) gradually increase over time; ATI and RI were significantly higher than those of the healthy controls. At baseline, mean arterial blood flow velocity (BFVa) and mean arterial retinal blood flow (RBFa) in CM eyes were significantly higher than those in the healthy control group. BFVa and RBFa showed a decreasing trend over time after PRT. In addition, some retinal oxygen kinetics and hemodynamic indicators were also correlated with tumor size, patient gender, and age.
Conclusion: CM patients after iodine-125 plaque radiotherapy had significant retinal and vascular changes. Future research should focus on rapidly screening radiation microvascular complications and exploring more timely and effective interventions to protect visual function in CM patients.
Keywords: choroid melanoma; hemodynamics; plaque radiotherapy; retinal oxygen kinetics.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Wide-Field (15 × 9 mm) Swept-Source Optical Coherence Tomography Angiography Following Plaque Radiotherapy of Choroidal Melanoma: An Analysis of 105 eyes.Asia Pac J Ophthalmol (Phila). 2020 Jul-Aug;9(4):326-334. doi: 10.1097/APO.0000000000000282. Asia Pac J Ophthalmol (Phila). 2020. PMID: 32371740
-
Plaque radiotherapy for choroidal melanoma encircling the optic disc (circumpapillary choroidal melanoma).Arch Ophthalmol. 2007 Sep;125(9):1202-9. doi: 10.1001/archopht.125.9.1202. Arch Ophthalmol. 2007. PMID: 17846359
-
Intraoperative echographic localization of iodine-125 episcleral plaque for brachytherapy of choroidal melanoma.Am J Ophthalmol. 2000 Feb;129(2):199-204. doi: 10.1016/s0002-9394(99)00315-3. Am J Ophthalmol. 2000. PMID: 10682973
-
Ocular complications following I-125 brachytherapy for choroidal melanoma.Eye (Lond). 2009 Jun;23(6):1254-68. doi: 10.1038/eye.2009.43. Epub 2009 Mar 6. Eye (Lond). 2009. PMID: 19265865 Review.
-
[A new approach for studying the retinal and choroidal circulation].Nippon Ganka Gakkai Zasshi. 2004 Dec;108(12):836-61; discussion 862. Nippon Ganka Gakkai Zasshi. 2004. PMID: 15656089 Review. Japanese.
Cited by
-
The role of the tumour microenvironment in lung cancer and its therapeutic implications.Med Oncol. 2025 May 23;42(6):219. doi: 10.1007/s12032-025-02765-7. Med Oncol. 2025. PMID: 40407951 Free PMC article. Review.
References
-
- Rusňák Š., Hecová L., Kasl Z., Sobotová M., and Hauer L., “Therapy of Uveal Melanoma A Review,” Ceska a Slovenska Oftalmologie: Casopis Ceske Oftalmologicke Spolecnosti a Slovenske Oftalmologicke Spolecnosti 77, no. 1 (2020): 1–13. - PubMed
-
- Matet A., Daruich A., and Zografos L., “Radiation Maculopathy After Proton Beam Therapy for Uveal Melanoma: Optical Coherence Tomography Angiography Alterations Influencing Visual Acuity,” Investigative Ophthalmology & Visual Science 58, no. 10 (2017): 3851–3861. - PubMed
-
- Gündüz K., Shields C. L., Shields J. A., Cater J., Freire J. E., and Brady L. W., “Radiation Retinopathy Following Plaque Radiotherapy for Posterior Uveal Melanoma,” Archives of Ophthalmology 117, no. 5 (1999): 609–614. - PubMed
MeSH terms
Substances
Grants and funding
- BJZYQN-2023-25/The Fund for Beijing Science & Technology Development of Traditional Chinese Medicine
- Z201100005520045/Science & Technology Project of Beijing Municipal Science & Technology Commission
- 82220108017/National Natural Science Foundation of China
- 82141128/National Natural Science Foundation of China
- SZSM202311018/Sanming Project of Medicine in Shenzen Municipality
LinkOut - more resources
Full Text Sources
Medical
Research Materials

