Neutrophils in Tissue Injury and Repair: Molecular Mechanisms and Therapeutic Targets
- PMID: 40260014
- PMCID: PMC12010766
- DOI: 10.1002/mco2.70184
Neutrophils in Tissue Injury and Repair: Molecular Mechanisms and Therapeutic Targets
Abstract
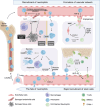
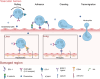
Tissue repair represents a highly intricate and ordered dynamic process, critically reliant on the orchestration of immune cells. Among these, neutrophils, the most abundant leukocytes in the body, emerge as the initial immune responders at injury sites. Traditionally recognized for their antimicrobial functions in innate immunity, neutrophils now garner attention for their indispensable roles in tissue repair. This review delves into their novel functions during the early stages of tissue injury. We elucidate the mechanisms underlying neutrophil recruitment and activation following tissue damage and explore their contributions to vascular network formation. Furthermore, we investigate the pivotal role of neutrophils during the initial phase of repair across different tissue types. Of particular interest is the investigation into how the fate of neutrophils influences overall tissue healing outcomes. By shedding light on these emerging aspects of neutrophil function in tissue repair, this review aims to pave the way for novel strategies and approaches in future organ defect repair, regeneration studies, and advancements in tissue engineering. The insights provided here have the potential to significantly impact the field of tissue repair and regeneration.
Keywords: angiogenesis; neutrophils; neutrophils fate; polarization; tissue repair.
© 2025 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Neutrophils in tissue injury and repair.Cell Tissue Res. 2018 Mar;371(3):531-539. doi: 10.1007/s00441-017-2785-7. Epub 2018 Jan 30. Cell Tissue Res. 2018. PMID: 29383445 Free PMC article. Review.
-
Neutrophils at the Crossroads: Unraveling the Multifaceted Role in the Tumor Microenvironment.Int J Mol Sci. 2024 Mar 2;25(5):2929. doi: 10.3390/ijms25052929. Int J Mol Sci. 2024. PMID: 38474175 Free PMC article. Review.
-
Electrospun fiber-based strategies for controlling early innate immune cell responses: Towards immunomodulatory mesh designs that facilitate robust tissue repair.Acta Biomater. 2023 Jun;163:228-247. doi: 10.1016/j.actbio.2022.06.004. Epub 2022 Jun 5. Acta Biomater. 2023. PMID: 35675893 Review.
-
More friend than foe: the emerging role of neutrophils in tissue repair.J Clin Invest. 2019 Jun 17;129(7):2629-2639. doi: 10.1172/JCI124616. eCollection 2019 Jun 17. J Clin Invest. 2019. PMID: 31205028 Free PMC article. Review.
-
Beyond host defense and tissue injury: the emerging role of neutrophils in tissue repair.Am J Physiol Cell Physiol. 2024 Mar 1;326(3):C661-C683. doi: 10.1152/ajpcell.00652.2023. Epub 2024 Jan 8. Am J Physiol Cell Physiol. 2024. PMID: 38189129 Free PMC article. Review.
Cited by
-
The role of multi-omics in biomarker discovery, diagnosis, prognosis, and therapeutic monitoring of tissue repair and regeneration processes.J Orthop Translat. 2025 Aug 8;54:131-151. doi: 10.1016/j.jot.2025.07.006. eCollection 2025 Sep. J Orthop Translat. 2025. PMID: 40822515 Free PMC article. Review.
-
Ferroptosis and Metabolic Dysregulation: Emerging Chemical Targets in Cancer and Infection.Molecules. 2025 Jul 18;30(14):3020. doi: 10.3390/molecules30143020. Molecules. 2025. PMID: 40733290 Free PMC article. Review.
-
A Review of Pathophysiology, Molecular Mechanisms, and Omics Approaches of Spinal Cord Injury.Int J Mol Sci. 2025 Aug 15;26(16):7895. doi: 10.3390/ijms26167895. Int J Mol Sci. 2025. PMID: 40869215 Free PMC article. Review.
-
Biomolecule-functionalized dental implant surfaces: Towards augmenting soft tissue integration.Bioact Mater. 2025 Jul 26;53:540-590. doi: 10.1016/j.bioactmat.2025.07.005. eCollection 2025 Nov. Bioact Mater. 2025. PMID: 40755849 Free PMC article. Review.
-
Feedback Loops Shape Oxidative and Immune Interactions in Hepatic Ischemia-Reperfusion Injury.Antioxidants (Basel). 2025 Jul 31;14(8):944. doi: 10.3390/antiox14080944. Antioxidants (Basel). 2025. PMID: 40867840 Free PMC article. Review.
References
-
- Shanley L. C., Mahon O. R., Kelly D. J., and Dunne A., “Harnessing the Innate and Adaptive Immune System for Tissue Repair and Regeneration: Considering More Than Macrophages,” Acta Biomaterialia 133 (2021): 208–221. - PubMed
-
- Mantovani A., Cassatella M. A., Costantini C., and Jaillon S., “Neutrophils in the Activation and Regulation of Innate and Adaptive Immunity,” Nature Reviews Immunology no. 8 (2011): 519–531. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
