A disintegrin-like and metalloproteinase 15 facilitates glioblastoma proliferation and metastasis through activation of the protease-activated receptor 1
- PMID: 40260066
- PMCID: PMC12010881
- DOI: 10.25259/Cytojournal_92_2024
A disintegrin-like and metalloproteinase 15 facilitates glioblastoma proliferation and metastasis through activation of the protease-activated receptor 1
Abstract
Objective: Glioblastoma hinders therapeutic interventions and prognostic outlooks. At the same time, a disintegrin-like and metalloproteinase 15 (ADAM15) influences cellular processes, such as adhesion and migration. Furthermore, protease-activated receptor 1 (PAR1), a vital receptor, impacts tumorigenesis and disease progression. This study aimed to investigate ADAM15 and PAR1 interaction in epithelial-mesenchymal transition (EMT) modulation in glioblastoma behavior and provide insights into therapeutic targets.
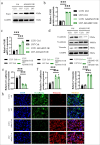
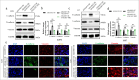
Material and methods: The impacts of ADAM15 overexpression and PAR-1/2 inhibition on the proliferation, invasion, and migration of glioblastoma cells U251 and U87 were evaluated using transwell assays, EdU incorporation, clonogenic assay, Ki67 immunohistochemistry, and immunofluorescence staining. Real-time quantitative polymerase chain reaction and Western blot analysis were employed to investigate the impact of ADAM15 on PAR1 expression.
Results: After analyzing the impacts of ADAM15 overexpression on the migration, invasion, and proliferation of human glioblastoma cell lines U251 and U87, the results showed that ADAM15 overexpression significantly enhanced migration (P < 0.001) and invasion rates (P < 0.001), as confirmed by scratch and transwell assays, thus indicating its tumor-promoting effects. This study revealed a significant increase in colony formation (P < 0.001), EdU incorporation (P < 0.001), and Ki67-positive cells (P < 0.001) in the ADAM15 overexpressed group. PAR1 and EMT markers were significantly increased in the ADAM15 overexpressed group (P < 0.001). Treatment with the PAR-1 antagonist SCH79797 inhibited EMT (P < 0.01) and suppressed cell proliferation (P < 0.001), migration (P < 0.001), and invasion (P < 0.001) in U251 and U87 cells overexpressing ADAM15, indicating the involvement of PAR-1 signaling in the effects of ADAM15 on cell behaviors. In comparison, the PAR-2 antagonist FSLLRY-NH2 did not show significant effects on EMT or these cell behaviors.
Conclusion: ADAM15 drives glioblastoma cell lines U251 and U87 progression through PAR1.
Keywords: A disintegrin-like and metalloproteinase domain 15; Epithelial-mesenchymal transition; Protease-activated receptor 1; glioblastoma.
© 2025 The Author(s). Published by Scientific Scholar.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Ethyl pyruvate inhibits glioblastoma cells migration and invasion through modulation of NF-κB and ERK-mediated EMT.PeerJ. 2020 Jul 21;8:e9559. doi: 10.7717/peerj.9559. eCollection 2020. PeerJ. 2020. PMID: 32742812 Free PMC article.
-
KLK8 promotes the proliferation and metastasis of colorectal cancer via the activation of EMT associated with PAR1.Cell Death Dis. 2021 Sep 22;12(10):860. doi: 10.1038/s41419-021-04149-x. Cell Death Dis. 2021. PMID: 34552064 Free PMC article.
-
Norepinephrine inhibits migration and invasion of human glioblastoma cell cultures possibly via MMP-11 inhibition.Brain Res. 2021 Apr 1;1756:147280. doi: 10.1016/j.brainres.2021.147280. Epub 2021 Jan 27. Brain Res. 2021. PMID: 33515535 Free PMC article.
-
Sinomenine Hydrochloride Inhibits the Metastasis of Human Glioblastoma Cells by Suppressing the Expression of Matrix Metalloproteinase-2/-9 and Reversing the Endogenous and Exogenous Epithelial-Mesenchymal Transition.Int J Mol Sci. 2018 Mar 14;19(3):844. doi: 10.3390/ijms19030844. Int J Mol Sci. 2018. PMID: 29538296 Free PMC article.
-
The role of the disintegrin metalloproteinase ADAM15 in prostate cancer progression.J Cell Biochem. 2009 Apr 15;106(6):967-74. doi: 10.1002/jcb.22087. J Cell Biochem. 2009. PMID: 19229865 Review.
References
LinkOut - more resources
Full Text Sources
