Enhanced efficacy of dual chimeric antigen receptor-T cells targeting programmed death-ligand 1 and cancer-associated fibroblasts in colorectal cancer in vitro
- PMID: 40260068
- PMCID: PMC12010817
- DOI: 10.25259/Cytojournal_245_2024
Enhanced efficacy of dual chimeric antigen receptor-T cells targeting programmed death-ligand 1 and cancer-associated fibroblasts in colorectal cancer in vitro
Abstract
Objective: Colorectal cancer (CRC) presents significant treatment challenges, including immune evasion and tumor microenvironment (TME) suppression. Chimeric antigen receptor (CAR) T-cell therapy has shown promise in hematologic malignancies, but its effectiveness against solid tumors is hampered by the detrimental effects of the TME. This article aims to explore the potential of bispecific CAR T cells targeting programmed death-ligand 1 (PD-L1) and cancer-associated fibroblasts (CAFs) in CRC treatment.
Material and methods: Dual-targeted CAR-T cells against PD-L1 and CAF were engineered using the GV400 lentiviral vector. Programmed death-1 (PD-1)/nanobody (Nb) and fibroblast activation protein (FAP)/Nb-encoding lentiviral vectors were generated, and CAR T cells were produced through a three-plasmid system in 293T cells. Human peripheral blood mononuclear cells (PBMCs) were separated, transduced with these vectors, and then expanded. Functional characterization of CAR-T cells was performed through enzyme-linked immunosorbent assay (ELISA), Western blot analysis, flow cytometry, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays, and cell counting kit-8 (CCK-8) assay. Migration and invasion assays were conducted using Transwell chambers to assess the ability of FAP-PD-1/Nb CAR-T cells to migrate toward tumor cells and invade the extracellular matrix.
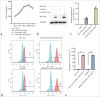
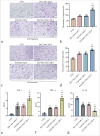
Results: We developed dual-targeted CAR-T cells incorporating PD-L1 and CAF Nbs, which continuously secreted PD-1/Nb. Western blot confirmed PD-1/Nb expression in PD-1/Nb and FAP-PD-1/Nb CAR-T cells, with no expression in the untreated (UTD) group (P < 0.01). Flow cytometry showed a significantly higher cluster of differentiation (CD)25 and CD69 expression in FAP-PD-1/Nb CAR-T cells upon stimulation with FAP-positive target cells compared with the other groups (P < 0.01). TUNEL, flow cytometry, and CCK-8 assays revealed that FAP-PD-1/Nb CAR-T cells exhibited superior cytotoxicity and proliferation inhibition against FAP-positive HCT116 cells (P < 0.01). ELISA demonstrated increased interferon-gamma and tumor necrosis factor-alpha levels and reduced interleukin-10 (P < 0.01), suggesting enhanced cytokine modulation and antitumor immunity. Compared with single-target CAR-T cells and UTD, FAP-PD-1/Nb CAR-T cells showed notably enhanced Matrigel penetration and invasion (P < 0.01). Safety tests confirmed minimal cytotoxicity to normal PBMCs, indicating favorable safety.
Conclusion: This study successfully developed dual-targeted CAR-T cells against PD-L1 and CAF and demonstrated their superior antitumor activity and immunomodulatory effects on CRC treatment. This novel therapeutic strategy was established using CAR T-cell technology for the treatment of CRC.
Keywords: Chimeric antigen receptor-T cells; Colorectal cancer; Fibroblast activation protein; Programmed death-ligand 1; Tumor microenvironment.
© 2025 The Author(s). Published by Scientific Scholar.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Dual-function chimeric antigen receptor T cells targeting c-Met and PD-1 exhibit potent anti-tumor efficacy in solid tumors.Invest New Drugs. 2021 Feb;39(1):34-51. doi: 10.1007/s10637-020-00978-3. Epub 2020 Aug 8. Invest New Drugs. 2021. PMID: 32772342
-
Stromal depletion by TALEN-edited universal hypoimmunogenic FAP-CAR T cells enables infiltration and anti-tumor cytotoxicity of tumor antigen-targeted CAR-T immunotherapy.Front Immunol. 2023 May 12;14:1172681. doi: 10.3389/fimmu.2023.1172681. eCollection 2023. Front Immunol. 2023. PMID: 37251405 Free PMC article.
-
PD-L1 chimeric costimulatory receptor improves the efficacy of CAR-T cells for PD-L1-positive solid tumors and reduces toxicity in vivo.Biomark Res. 2020 Nov 2;8(1):57. doi: 10.1186/s40364-020-00237-w. Biomark Res. 2020. PMID: 33292688 Free PMC article.
-
Fibroblast Activation Protein (FAP)-Targeted CAR-T Cells: Launching an Attack on Tumor Stroma.Immunotargets Ther. 2021 Aug 5;10:313-323. doi: 10.2147/ITT.S291767. eCollection 2021. Immunotargets Ther. 2021. PMID: 34386436 Free PMC article. Review.
-
The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment.Bioact Mater. 2020 Dec 26;6(7):1973-1987. doi: 10.1016/j.bioactmat.2020.12.010. eCollection 2021 Jul. Bioact Mater. 2020. PMID: 33426371 Free PMC article. Review.
Cited by
-
Paracrine signaling in cancer-associated fibroblasts: central regulators of the tumor immune microenvironment.J Transl Med. 2025 Jun 23;23(1):697. doi: 10.1186/s12967-025-06744-4. J Transl Med. 2025. PMID: 40551209 Free PMC article. Review.
References
-
- Peng C, Xiong F, Pu X, Hu Z, Yang Y, Qiao X, et al. m(6) A methylation modification and immune cell infiltration: implications for targeting the catalytic subunit m(6)A-METTL complex in gastrointestinal cancer immunotherapy. Front Immunol. 2023;14:1326031. doi: 10.3389/fimmu.2023.1326031. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
