Optimized Effects of Fisetin and Hydroxychloroquine on ER Stress and Autophagy in Nonalcoholic Fatty Pancreas Disease in Mice
- PMID: 40260275
- PMCID: PMC12011465
- DOI: 10.1155/jdr/2795127
Optimized Effects of Fisetin and Hydroxychloroquine on ER Stress and Autophagy in Nonalcoholic Fatty Pancreas Disease in Mice
Abstract
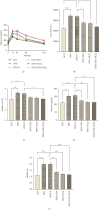
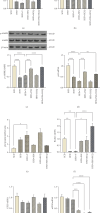
Background: Fat accumulation in the pancreas, known as nonalcoholic fatty pancreatic disease (NAFPD), is associated with obesity and may lead to prediabetes and Type 2 diabetes. Reducing endoplasmic reticulum stress and enhancing autophagy could offer therapeutic benefits. This study examines the effects of fisetin (FSN) and hydroxychloroquine (HCQ) on NAFPD. Method: Forty-eight Male C57BL/6 J mice were assigned to a standard chow diet (SCD) or a high-fat diet (HFD) for 16 weeks. The HFD group was divided into five subgroups; each group contains eight mice: HFD, HFD + V (vehicle), HFD + FSN, HFD + HCQ, and HFD + FSN + HCQ. FSN was given daily at 80 mg/kg, and HCQ was injected IP at 50 mg/kg twice weekly for more 8 weeks. Insulin resistance was assessed through OGTT and HOMA-IR. Histological analysis of pancreatic tissue was conducted, and the protein and mRNA levels of molecules associated with ER stress and autophagy were assessed using PCR and immunoblotting techniques. Result: FSN and HCQ significantly reduced weight gain, pancreatic adipocyte accumulation, and insulin resistance caused by HFD in obese mice, with the combination of the two compounds producing even more pronounced effects. Additionally, the HFD increased the expression of UPR markers ATF4 and CHOP, a response that was further intensified by HCQ. In contrast, FSN attenuated the UPR by regulating GRP78 levels. Furthermore, the HFD resulted in a significant decrease in the LC3II/LC3I ratio and an accumulation of p62 protein due to reduced p-AMPK levels. Following treatment with FSN, these alterations were reversed, leading to decreased mTOR expression and increased levels of autophagy markers such as ATG5 and Beclin1. Conclusion: Our study reveals that FSN and HCQ effectively combat HFD-induced NAFPD, improving insulin sensitivity and addressing pancreatic fat deposition linked to metabolic syndrome. While HCQ may cause endoplasmic reticulum stress, FSN offers protective effects, supporting their combined use for better treatment outcomes.
Keywords: ER stress; autophagy; fatty pancreas; fisetin; hydroxychloroquine; metabolic syndrome.
Copyright © 2025 Mahboobe Sattari et al. Journal of Diabetes Research published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Therapeutic potential of fisetin in hepatic steatosis: Insights into autophagy pathway regulation and endoplasmic reticulum stress alleviation in high-fat diet-fed mice.PLoS One. 2025 May 22;20(5):e0322335. doi: 10.1371/journal.pone.0322335. eCollection 2025. PLoS One. 2025. PMID: 40402993 Free PMC article.
-
Empagliflozin Attenuates Non-Alcoholic Fatty Liver Disease (NAFLD) in High Fat Diet Fed ApoE(-/-) Mice by Activating Autophagy and Reducing ER Stress and Apoptosis.Int J Mol Sci. 2021 Jan 15;22(2):818. doi: 10.3390/ijms22020818. Int J Mol Sci. 2021. PMID: 33467546 Free PMC article.
-
Improving Non-alcoholic Fatty Liver Disease Treatment in High-fat Diet Fed Mice with Fisetin and Hydroxychloroquine: The Cooperative Pathways for Improved Metabolic Health.Curr Med Chem. 2025 Feb 11. doi: 10.2174/0109298673342421241126060049. Online ahead of print. Curr Med Chem. 2025. PMID: 39931978
-
[Effects of celastrol on autophagy and endoplasmic reticulum stress-mediated apoptosis in a mouse model of nonalcoholic fatty liver disease].Zhonghua Gan Zang Bing Za Zhi. 2022 Jun 20;30(6):656-662. doi: 10.3760/cma.j.cn501113-20210817-00408. Zhonghua Gan Zang Bing Za Zhi. 2022. PMID: 36038329 Chinese.
-
α-Linolenic Acid-Enriched Cold-Pressed Perilla Oil Suppress High-Fat Diet-Induced Hepatic Steatosis through Amelioration of the ER Stress-Mediated Autophagy.Molecules. 2020 Jun 8;25(11):2662. doi: 10.3390/molecules25112662. Molecules. 2020. PMID: 32521713 Free PMC article.
References
-
- Lesmana C. R., Pakasi L. S., Inggriani S., Aidawati M. L., Lesmana L. A. Prevalence of Non-Alcoholic Fatty Pancreas Disease (NAFPD) and Its Risk Factors Among Adult Medical Check-Up Patients in a Private Hospital: A Large Cross Sectional Study. BMC Gastroenterology . 2015;15(1):p. 174. doi: 10.1186/s12876-015-0404-1. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

