Efficacy and Safety of Mirikizumab in the Treatment of Moderately to Severely Active Ulcerative Colitis Regardless of Baseline Modified Mayo Score: Results From the Phase 3 LUCENT Trials
- PMID: 40260308
- PMCID: PMC12010086
- DOI: 10.1093/crocol/otaf002
Efficacy and Safety of Mirikizumab in the Treatment of Moderately to Severely Active Ulcerative Colitis Regardless of Baseline Modified Mayo Score: Results From the Phase 3 LUCENT Trials
Abstract
Background: The modified Mayo score (mMS) is a measure for ulcerative colitis (UC) disease activity. Recent US Food and Drug Administration guidance for moderately to severely active UC trials suggests that patients should have baseline mMS of 5-9 including an endoscopy score of at least 2, as opposed to the previous range of 4-9. This disclosure reports results from patients with UC with baseline mMS of 5-9 who received mirikizumab, a monoclonal antibody directed against the interleukin-23 p19 subunit, or placebo in the phase 3 LUCENT trials.
Methods: Mirikizumab was evaluated in the randomized, double-blind, placebo-controlled LUCENT-1 (NCT03518086) and LUCENT-2 (NCT03524092) trials, and the ongoing long-term LUCENT-3 (NCT03519945) trial, which use mMS 4-9. Analyses for patients with baseline mMS of 5-9 (excluding patients with mMS of 4) were conducted according to LUCENT trial statistical analysis plans. Categorical efficacy endpoints were summarized using proportions and confidence intervals. Continuous efficacy endpoints are presented as least-squares mean (standard error) changes from baseline.
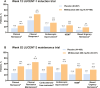
Results: Mirikizumab demonstrated efficacy for the primary endpoint of clinical remission and major secondary endpoints including clinical response, endoscopic improvement, histologic-endoscopic mucosal improvement/remission, bowel urgency remission, and corticosteroid-free remission. Importantly, mirikizumab exhibited greater improvements versus placebo in the Inflammatory Bowel Disease Questionnaire, fatigue, symptomatic remission, and work productivity. Finally, mirikizumab demonstrated long-term (104-week) sustained, durable efficacy across all studied endpoints. No new safety signals were identified during the 2-year follow-up.
Conclusions: Mirikizumab delivered significant clinical benefit for patients with baseline mMS of 5-9 and demonstrated a favorable safety profile.
Keywords: fatigue; mirikizumab; modified Mayo score; ulcerative colitis; urgency.
© The Author(s) 2025. Published by Oxford University Press on behalf of Crohn's & Colitis Foundation.
Conflict of interest statement
A.D. holds the position of Deputy Editor for Crohn’s & Colitis 360 and has been recused from reviewing or making decisions for the manuscript. Dr. Charabaty is also a consultant and advisory board member for AbbVie, Eli Lilly and Company, Janssen, Pfizer, and Takeda. E.L.B. is a consultant for AbbVie. A.C.E. is a consultant and speaker for AbbVie, Bristol Myers Squibb, and Eli Lilly and Company; consultant for Janssen; and speaker for Nestlé/Aimmune Therapeutics.
Figures





References
-
- Irvine EJ. Quality of life of patients with ulcerative colitis: past, present, and future. Inflamm Bowel Dis. 2008;14(4):554-565. doi: https://doi.org/ 10.1002/ibd.20301 - DOI - PubMed
-
- Dubinsky M, Panaccione R, Lopez-Sanroman A, et al. P024 patient and healthcare provider views on ulcerative colitis treatment goals and quality of life: results of a global ulcerative colitis narrative survey. Gastroenterology. 2019;156(3):S17-S18. doi: https://doi.org/ 10.1053/j.gastro.2019.01.073 - DOI
-
- United States Food and Drug Administration. Ulcerative Colitis: Clinical Trial Endpoints Guidance for Industry. August 2016.
-
- United States Food and Drug Administration. Ulcerative Colitis: Developing Drugs for Treatment Guidance for Industry. April 2022.
-
- Sandborn WJ, Sands BE, Vermeire S, et al. Modified Mayo score versus Mayo score for evaluation of treatment efficacy in patients with ulcerative colitis: data from the tofacitinib OCTAVE program. Therap Adv Gastroenterol 2022;15:17562848221136331. doi: https://doi.org/ 10.1177/17562848221136331 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
