Lactoferrin-derived peptide PXL01 impacts nerve regeneration after sciatic nerve reconstruction in healthy and diabetic rats
- PMID: 40260416
- PMCID: PMC12009942
- DOI: 10.3389/fcell.2025.1565285
Lactoferrin-derived peptide PXL01 impacts nerve regeneration after sciatic nerve reconstruction in healthy and diabetic rats
Abstract
Introduction: Although advanced surgical techniques are available, satisfactory functional outcomes after peripheral nerve injuries are uncommon. Hence, immune-modulating factors such as PXL01, a lactoferrin-derived peptide that improves axonal outgrowth in injured human digital nerves, have gained attention. We previously reported a short-term immunosuppressive effect of PXL01 after the repair of transected rat sciatic nerves, but it had no effect on nerve regeneration. Here, we investigated the potential of PXL01 to improve nerve regeneration in healthy rats and in a rat model of type 2 diabetes (Goto-Kakizaki [GK] rats).
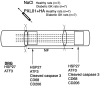
Methods: A 10-mm sciatic nerve defect was created in healthy (n = 14) and diabetic GK rats (n = 14) and reconstructed using nerve autografts. Immediately after surgery, PXL01 or sodium chloride (control, placebo) (n = 7 for each treatment) was administered around the autograft. On day 8, immunohistochemical staining of the sciatic nerve and dorsal root ganglia (DRGs) was performed to analyze axonal outgrowth (neurofilament staining); inflammation (CD68 and CD206 macrophage staining in nerve); Schwann cell and sensory neuron activation (transcription factor ATF3 staining in nerve and DRGs) and apoptosis (cleaved caspase 3 staining in nerve); and neuroprotection (heat shock protein [HSP27] staining in nerve and DRGs).
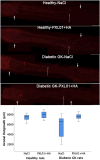
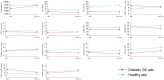
Results: PXL01 had no impact on the macrophage response in the autografts but increased axonal outgrowth and HSP27 expression in the DRGs of healthy and diabetic rats, despite a lower number of activated Schwann cells in the autograft. Diabetes affected axonal outgrowth, Schwann cell and macrophage responses, and HSP27 expression. These effects were observed in the sciatic nerve as well as the DRG.
Discussion: Application of PXL01, despite having no impact on macrophages, may improve axonal outgrowth and affects Schwann cell activation in autograft-reconstructed sciatic nerves, as well as conveys neuroprotection (HSP27 expression) in the DRGs of healthy and diabetic GK rats. Diabetes influenced nerve regeneration in such autografts. Therefore, PXL01 is a promising candidate to improve nerve regeneration.
Keywords: Goto-Kakizaki rat; PXL01; autograft; axonal outgrowth; diabetes; nerve reconstruction; nerve regeneration.
Copyright © 2025 Hazer Rosberg, Mahlapuu, Perez and Dahlin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
PXL01 alters macrophage response with no effect on axonal outgrowth or Schwann cell response after nerve repair in rats.Regen Med. 2024 Jun 2;19(6):327-343. doi: 10.1080/17460751.2024.2361515. Epub 2024 Jul 3. Regen Med. 2024. PMID: 38957920 Free PMC article.
-
Gender differences in nerve regeneration after sciatic nerve injury and repair in healthy and in type 2 diabetic Goto-Kakizaki rats.BMC Neurosci. 2014 Sep 13;15:107. doi: 10.1186/1471-2202-15-107. BMC Neurosci. 2014. PMID: 25216784 Free PMC article.
-
Injury-Induced HSP27 Expression in Peripheral Nervous Tissue Is Not Associated with Any Alteration in Axonal Outgrowth after Immediate or Delayed Nerve Repair.Int J Mol Sci. 2021 Aug 11;22(16):8624. doi: 10.3390/ijms22168624. Int J Mol Sci. 2021. PMID: 34445330 Free PMC article.
-
Impaired Axonal Regeneration in Diabetes. Perspective on the Underlying Mechanism from In Vivo and In Vitro Experimental Studies.Front Endocrinol (Lausanne). 2017 Feb 1;8:12. doi: 10.3389/fendo.2017.00012. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28203223 Free PMC article. Review.
-
The Dynamics of Nerve Degeneration and Regeneration in a Healthy Milieu and in Diabetes.Int J Mol Sci. 2023 Oct 16;24(20):15241. doi: 10.3390/ijms242015241. Int J Mol Sci. 2023. PMID: 37894921 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

