TTR Gene Screening Since the Advent of Biotherapies in France: A Nationwide Retrospective Survey Between 2018 and 2023
- PMID: 40260709
- PMCID: PMC12012641
- DOI: 10.1111/ene.70104
TTR Gene Screening Since the Advent of Biotherapies in France: A Nationwide Retrospective Survey Between 2018 and 2023
Abstract
Background: Hereditary transthyretin amyloidosis (ATTRv) is a rare genetic disorder caused by mutations in the TTR gene. Associated with various clinical phenotypes like polyneuropathy and cardiomyopathy, ATTRv has historically had poor outcomes. Recent advances in biotherapies have significantly improved these outcomes. This study aimed to assess the evolution in genetic TTR variant screening since the advent of biotherapies in France in 2018.
Methods: This nationwide retrospective study analyzed data and genetic results from patients who underwent TTR gene sequencing from 2018 to 2023.
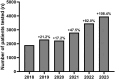
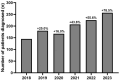
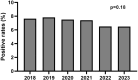
Results: 16,640 patients were tested during the period studied. There was a 108% increase in the number of TTR gene sequencing performed annually between 2018 and 2023. Positive rates remained stable despite increased testing (7.09% over time). During this 6-year period, 1,179 patients were diagnosed with a pathogenic variant of TTR.
Conclusions: The study shows a substantial rise in TTR genetic testing in France, likely linked to the deployment of biotherapies. These findings underscore the necessity of integrating TTR gene sequencing into standard diagnostic procedures, especially given the effectiveness of treatments and the stability of positive rates.
Keywords: TTR gene; amyloidosis; epidemiology; nationwide survey; transthyretin.
© 2025 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
Prof. Echaniz‐Laguna received consulting fees from Alnylam Pharmaceuticals, Akcea Therapeutics, AstraZeneca, and Pfizer. Docteur Labeyrie received consulting fees and a speech honorarium from Alnylam Pharmaceuticals and Pfizer. Dr. Cauquil received consulting fees and a speech honorarium from Pfizer, Alnylam Pharmaceuticals, and AstraZeneca. Pr. Adams received consulting fees from Pfizer, Alnylam, Astrazeneca, BridgeBio, and payment of transport costs for the ISA congress from Alnylam. A.E.K.A.T., P.C., V.P., C.B., C.‐M.D., B.F., C.L.R., G.J., A.‐S.L., M.L., A.P., F.S., L.T., C.V., and J.B. declare no conflicts of interest.
Figures
References
-
- Fargeot G., Echaniz‐Laguna A., Labeyrie C., et al., “Hereditary Transthyretin Amyloidosis in Middle‐Aged and Elderly Patients With Idiopathic Polyneuropathy: A Nationwide Prospective Study,” Amyloid 31 (2023): 1–8. - PubMed
-
- Wechalekar A. D., Gillmore J. D., and Hawkins P. N., “Systemic Amyloidosis,” Lancet 387, no. 10038 (2016): 2641–2654. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

