Hemodynamics and Drinking in the Giraffe
- PMID: 40260757
- PMCID: PMC12012874
- DOI: 10.1111/apha.70046
Hemodynamics and Drinking in the Giraffe
Abstract
Background: The circulation of 4-6 m tall giraffes is markedly affected by gravity. To ensure cerebral perfusion, upright giraffes generate a blood pressure in excess of 200 mmHg. Before drinking, the head is lowered by 3-5 m, providing exceptional hemodynamic challenges. Here, we provide quantitative hemodynamic measures during head movement and drinking.
Methods: We measured carotid pressure, jugular pressure, heart rate, and blood flow in awake giraffes, along with circulating blood volume and cerebrospinal fluid pressure in anesthetized giraffes. We also analyzed the contractility and innervation of isolated cerebral and extracranial arteries, and the mechanical properties of jugular veins.
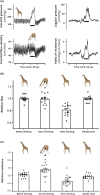
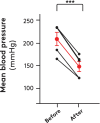
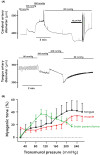
Results: When heads were lowered for drinking (i) blood pressure at heart level decreased but increased again during drinking, (ii) jugular pressure increased and oscillated during drinking, (iii) heart rate fell, (iv) carotid blood flow was unchanged, while cephalic hemodynamic resistance increased, and (vi) cranial cerebrospinal fluid pressure increased. Small cerebral arteries exhibited strong myogenic responses, particularly at around 100 mmHg, while extracranial arteries responded at higher pressures (200-250 mmHg). The giraffe's blood volume was small and blood pressure sensitive to minor reductions in blood volume.
Conclusions: Central blood pressure decreased when the head was lowered, but drinking per se caused a surprising rise in blood pressure to pre-drinking levels. This rise in blood pressure is likely due to the transfer of esophageal water boli acting on the jugular veins. The cephalic capillaries are protected by a strong myogenic response and sympathetic innervation.
Keywords: blood pressure; giraffes; gravitational physiology; hypertension.
© 2025 The Author(s). Acta Physiologica published by John Wiley & Sons Ltd on behalf of Scandinavian Physiological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Goetz R. H., “Preliminary Observations of the Circulation in the Giraffe,” Transactions of the American College of Cardiology 5 (1955): 239–248. - PubMed
-
- Hargens A. R., Millard R. W., Pettersson K., and Johansen K., “Gravitational Haemodynamics and Oedema Prevention in the Giraffe,” Nature 329, no. 6134 (1987): 59–60. - PubMed
-
- Lawrence W. E. and Rewell R. E., “The Cerebral Blood Supply in the Giraffidae,” Proceedings of the Zoological Society 118 (1948): 202–212.
-
- Mitchell G. and Skinner J. D., “An Allometric Analysis of the Giraffe Cardiovascular System,” Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology 154, no. 4 (2009): 523–529. - PubMed
-
- Van Citters R. L., Franklin D. L., Vatner S. F., Patrick T., and Warren J. V., “Cerebral Hemodynamics in the Giraffe,” Transactions of the Association of American Physicians 82 (1969): 293–304. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

