Circulating Tumour DNA as a Complementary Tool for Treatment Evaluation in HPV-Associated Head and Neck Squamous Cell Carcinoma: An Observational Cohort Study
- PMID: 40260766
- PMCID: PMC12319461
- DOI: 10.1111/coa.14317
Circulating Tumour DNA as a Complementary Tool for Treatment Evaluation in HPV-Associated Head and Neck Squamous Cell Carcinoma: An Observational Cohort Study
Abstract
Objectives: HPV-positive oropharyngeal squamous cell carcinoma (OPSCC) and head and neck carcinoma of unknown primary (HNCUP) are increasing. Despite good prognosis, recurrence rates range from 10% to 25%. Surveillance with clinical controls and imaging is not always reliable. Circulating tumour human papillomavirus DNA (ctHPV-DNA) has emerged as a potential biomarker for treatment evaluation and detection of recurrence. We aimed to investigate the correlation between ctHPV-DNA in HPV+ OPSCC/HNCUP and radiologic tumour burden. Additionally, we sought to assess whether ctHPV-DNA could serve as a tool in treatment evaluation.
Design: A prospective observational cohort study.
Setting: This multicenter study involved three otolaryngology units located in central Sweden. We utilised HPV genotype-specific assays for droplet digital PCR (ddPCR) to detect ctHPV-DNA in plasma at diagnosis and follow-up. ctHPV-DNA levels were correlated to radiological tumour burden and radiological response using the Kendall Rank correlation coefficient and the Kruskal-Wallis test.
Participants: Patients with HPV+ OPSCC/HNCUP undergoing definitive (chemo)radiotherapy and enrolled in the CIRCOS study.
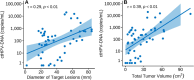
Results: Out of 54 patients, 51 were eligible for analyses. At baseline, ctHPV-DNA was detectable in 88%. A majority of patients with a favourable radiological evaluation according to RECIST had a corresponding undetectable ctHPV-DNA at follow-up. The levels of ctHPV-DNA at baseline correlated with total tumour volume and nodal volume (rτ = 0.39, p < 0.01, respectively rτ = 0.26, p < 0.01).
Conclusion: ctHPV-DNA shows correlation with tumour burden. This study strengthens the role of ctHPV-DNA as a promising biomarker for treatment evaluation in HPV-related OPC/HNCUP. With further research on serial plasma sampling, ctHPV-DNA could complement radiological treatment evaluation in HPV+ OPSCC/HNCUP.
Trial registration: NCT05904327 [ClinicalTrials.gov].
Keywords: RECIST; biomarker; cancer of unknown primary; ctHPV‐DNA; head and neck squamous cell carcinoma; human papilloma virus; oropharyngeal cancer.
© 2025 The Author(s). Clinical Otolaryngology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Zamani M., Grønhøj C., Jensen D. H., et al., “The Current Epidemic of HPV‐Associated Oropharyngeal Cancer: An 18‐Year Danish Population‐Based Study With 2,169 Patients,” European Journal of Cancer 134 (2020): 52–59. - PubMed
-
- Garset‐Zamani M., Carlander A. F., Jakobsen K. K., et al., “Impact of Specific High‐Risk Human Papillomavirus Genotypes on Survival in Oropharyngeal Cancer,” International Journal of Cancer 150, no. 7 (2022): 1174–1183. - PubMed
-
- Mehanna H., Taberna M., von Buchwald C., et al., “Prognostic Implications of p16 and HPV Discordance in Oropharyngeal Cancer (HNCIG‐EPIC‐OPC): A Multicentre, Multinational, Individual Patient Data Analysis,” Lancet Oncology 24, no. 3 (2023): 239–251. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

