B cell lines fail to support efficient rhesus enteric calicivirus and human norovirus replication
- PMID: 40261012
- PMCID: PMC12090725
- DOI: 10.1128/jvi.00143-25
B cell lines fail to support efficient rhesus enteric calicivirus and human norovirus replication
Abstract
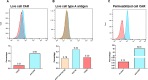
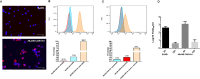
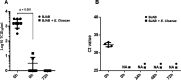
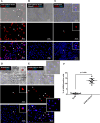
Analyses of intestinal biopsies of infected individuals and/or nonhuman primates (NHP) suggested the possible immune cell tropism of human noroviruses (HuNoV) and rhesus enteric caliciviruses (ReCV). Subsequently, the first HuNoV cell culture system using human B cell lines was reported. However, reproducibility issues raised questions about the validity and suitability of B cell cultures for HuNoV research. Histo-blood group antigens (HBGA) are known HuNoV susceptibility factors, but the full range of HuNoV susceptibility determinants remains unknown. In contrast, strain-specific ReCV susceptibility determinants have been recently characterized. Here, we evaluated NHP B cell lines and the human BJAB cell line for susceptibility to ReCV-FT285 infection, which is controlled by the Coxsackie and adenovirus receptor (CAR) and the type A or B HBGA. NHP B cell lines lacked CAR and HBGA expression and resisted infection. Inconsistent, low-level virus replication was detectable in BJAB cells, and expression of CAR and HBGAs was evident by Western blots. However, <1% of live, but >80% of fixed and permeabilized BJAB cells were CAR+, suggesting that CAR is mostly internalized. Co-transfection of BJAB cells with hCAR and A enzyme expression vectors led to substantial surface CAR and type A HBGA expression but not to an increase in ReCV titers. dsRNA staining revealed initial ReCV and HuNoV infection in a few cells that most likely became abortive. Based on both the similarities between ReCV and HuNoV replication profiles and the results obtained in the present study, considering BJAB cells an efficient culture system for HuNoV research is not justified.IMPORTANCERecently, two human norovirus (HuNoV) cell culture systems have been developed-the B cell culture system and the enteroid culture system. While the enteroid cell culture system became widely used in HuNoV research, mainly due to reproducibility issues, the B cell culture system did not. Here, we used HuNoV and rhesus enteric caliciviruses (ReCV) to evaluate enteric calicivirus B cell infections, in correlation to cell surface molecular determinants that control the susceptibility to infection. These are fully characterized for ReCVs, but not for HuNoVs. We found that only few BJAB cells express the cell surface molecules necessary for ReCV infection and support low-level, initial ReCV and HuNoV infection, but virus replication is most likely abortive, with minimal progeny virus release. Our findings and the poor reproducibility indicate that the B cell culture system in its current form is unsuitable for ReCV or HuNoV research and does not represent an efficient valid cell culture system.
Keywords: B cell; Coxsackie and adenovirus receptor; histo-blood group antigen; rhesus enteric calicivirus.
Conflict of interest statement
The author declares no conflict of interest.
Figures



References
-
- Burke RM, Mattison CP, Pindyck T, Dahl RM, Rudd J, Bi D, Curns AT, Parashar U, Hall AJ. 2021. Burden of norovirus in the United States, as estimated based on administrative data: updates for medically attended illness and mortality, 2001-2015. Clin Infect Dis 73:e1–e8. doi: 10.1093/cid/ciaa438 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

