Two-photon Excitation of Bright Diaza[4]Helicenes for Isotropic and Circularly Polarized Emission
- PMID: 40261255
- PMCID: PMC12144873
- DOI: 10.1002/chem.202501212
Two-photon Excitation of Bright Diaza[4]Helicenes for Isotropic and Circularly Polarized Emission
Abstract
Helicenes are chiral organic dyes that are attracting growing attention due to the high tunability of their (chir)optical and electronic properties. In this work, a series of functionalized cationic diaza[4]helicenes based on dimethoxyquinacridinium (DMQA) scaffolds are presented. By merging branched N-alkyl side chains and triple para-functionalization with OMe groups, structures combine improved chiroptical responses (5x increase) and strong fluorescence quantum yields (Φf ≈ 70% in acetonitrile). An overall improved efficiency of the emission of circularly polarized light with BCPL values reaching 3.4 M-1 cm-1 is obtained. Additionally, two-photon excitation (2PE) studies were performed, showing good values of cross section (CS) at 810 nm. Interestingly, 2PE circularly polarized luminescence (CPL) spectra were acquired for the most performant derivatives (N-isopropyl and N-cyclohexyl); this type of measurement being usually challenging for organic molecular species. Finally, the viability of these compounds in single-photon (1PE) and 2PE microscopy is also shown.
Keywords: bright fluorophores; chiroptical properties; circularly polarized luminescence; microscopy; two‐photon excitation.
© 2025 The Author(s). Chemistry – A European Journal published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




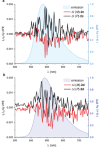
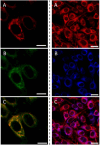
References
-
- a) Train C., Gruselle M., Verdaguer M., Chem. Soc. Rev. 2011, 40, 3297; - PubMed
- b) Mondal P. C., Fontanesi C., Waldeck D. H., Naaman R., Acc. Chem. Res. 2016, 49, 2560; - PMC - PubMed
- c) Han H., Lee Y. J., Kyhm J., Jeong J. S., Han J.‐H., Yang M. K., Lee K. M., Choi Y., Yoon T.‐H., Ju H., Ahn S.‐k., Lim J. A., Adv. Funct. Mater. 2020, 30, 2006236;
- d) Furlan F., Moreno‐Naranjo J. M., Gasparini N., Feldmann S., Wade J., Fuchter M. J., Nat. Photonics 2024, 18, 658;
- e) Artigas A., Ferdi N., Rémond M., Rigoulet F., Vanthuyne N., Hagebaum‐Reignier D., Carissan Y., Naubron J.‐V., Giorgi M., Favereau L., Coquerel Y., J. Org. Chem. 2024, 89, 498; - PubMed
- f) Guo W. C., Zhao W. L., Tan K. K., Li M., Chen C. F., Angew. Chem. 2024, 136, e202401835; - PubMed
- g) Zhang F., Brancaccio V., Saal F., Deori U., Radacki K., Braunschweig H., Rajamalli P., Ravat P., J. Am. Chem. Soc. 2024, 146, 29782; - PubMed
- h) Cadeddu S., van den Bersselaar B. W., de Waal B., Cordier M., Vanthuyne N., Meskers S. C., Vantomme G., Crassous J., Nanoscale 2024, 16, 21351; - PubMed
- i) Yin C., Ye X., Tao S., Zhao D., Zhi Y., Jiang D., Angew. Chem., Int. Ed. 2024, 63, e202411558; - PubMed
- j) He P., Ye J., Zhang J., Lu T., Cui W., Liu J., Shen C., Hong W., Liu X., Angew. Chem., Int. Ed. 2025, 64, e202416319; - PubMed
- k) Zhou Z., Petrukhina M. A., Chem. Sci. 2025, 16, 468. - PMC - PubMed
-
- a) Hutskalova V., Prescimone A., Sparr C., Helv. Chim. Acta 2021, 104, e2100182;
- b) Crassous J., Stará I. G., Stary I., Helicenes: Synthesis, Properties, and Applications, John Wiley & Sons, 2022;
- c) Xiao X., Cheng Q., Bao S. T., Jin Z., Sun S., Jiang H., Steigerwald M. L., Nuckolls C., J. Am. Chem. Soc. 2022, 144, 20214; - PubMed
- d) Fu W., Pelliccioli V., von Geyso M., Redero P., Böhmer C., Simon M., Golz C., Alcarazo M., Adv. Mater. 2023, 35, 2211279; - PubMed
- e) Izquierdo‐García P., Fernández‐García J. M., Rivero S. M., Šámal M., Rybáček J., Bednárová L., Ramírez‐Barroso S., Ramírez F. J., Rodríguez R., Perles J., García‐Fresnadillo D., Crassous J., Casado J., Stará I. G., Martín N., J. Am. Chem. Soc. 2023, 145, 11599; - PMC - PubMed
- f) Guo S.‐M., Huh S., Coehlo M., Shen L., Pieters G., Baudoin O., Nat. Chem. 2023, 15, 872; - PMC - PubMed
- g) Zhang G., Zhang J., Tao Y., Gan F., Lin G., Liang J., Shen C., Zhang Y., Qiu H., Nat. Commun. 2024, 15, 5469; - PMC - PubMed
- h) Sun Z., Xu W., Qiu S., Ma Z., Li C., Zhang S., Wang H., Chem. Sci. 2024, 15, 1077; - PMC - PubMed
- i) Mutoh K., Abe J., Chem. Sci. 2024, 15, 13343; - PMC - PubMed
- j) Singh H. K., Bodzioch A., Pietrzak A., Kaszyński P., Chem. Commun. 2025, 61, 496; - PubMed
- k) Huang S., Wen H., Li Y., Qin W., Wang P., Lan Y., Jia S., Yan H., Nat. Commun. 2025, 16, 500; - PMC - PubMed
- l) Gisbert Y., Ovalle M., Stindt C. N., Costil R., Feringa B. L., Angew. Chem., Int. Ed. 2025, 64, e202416097; - PMC - PubMed
- m) Hano N., Zigon N., Kuppan B., Sturm L., Vanthuyne N., Pouget E., Nlate S., Bock H., Durola F., Avarvari N., Nanoscale 2025, 17, 5081; - PubMed
- n) Edlová T., Rybáček J., Cattey H., Vacek J., Bednárová L., Le Gendre P., Normand A. T., Stará I. G., Starý I., Angew. Chem. 2025, 137, e202414698. - PMC - PubMed
-
- a) Delgado I. H., Pascal S., Wallabregue A., Duwald R., Besnard C., Guénée L., Nançoz C., Vauthey E., Tovar R. C., Lunkley J. L., Muller G., Lacour J., Chem. Sci. 2016, 7, 4685; - PMC - PubMed
- b) Duwald R., Pascal S., Bosson J., Grass S., Besnard C., Bürgi T., Lacour J., Chem.‐Eur. J. 2017, 23, 13596; - PubMed
- c) Frédéric L., Fabri B., Guénée L., Zinna F., Di Bari L., Lacour J., Chem.‐Eur. J. 2022, 28, e202201853; - PMC - PubMed
- d) Fabri B., Funaioli T., Frédéric L., Elsner C., Bordignon E., Zinna F., Di Bari L., Pescitelli G., Lacour J., J. Am. Chem. Soc. 2024, 146, 8308. - PubMed
-
- a) Kiran V., Mathew S. P., Cohen S. R., Hernández Delgado I., Lacour J., Naaman R., Adv. Mater. 2016, 28, 1957; - PubMed
- b) Babič A., Pascal S., Duwald R., Moreau D., Lacour J., Allémann E., Adv. Funct. Mater. 2017, 27, 1701839;
- c) Hargenrader G. N., Weerasooriya R. B., Ilic S., Niklas J., Poluektov O. G., Glusac K. D., ACS Appl. Energy Mater. 2019, 2, 80;
- d) Mei L., Veleta J. M., Gianetti T. L., J. Am. Chem. Soc. 2020, 142, 12056; - PubMed
- e) Moutet J., Mills D., Hossain M. M., Gianetti T. L., Mater. Adv. 2022, 3, 216;
- f) Calogero F., Magagnano G., Potenti S., Pasca F., Fermi A., Gualandi A., Ceroni P., Bergamini G., Cozzi P. G., Chem. Sci. 2022, 13, 5973; - PMC - PubMed
- g) Shaikh A. C., Hossain M. M., Moutet J., Kumar A., Thompson B., Huxter V. M., Gianetti T. L., Angew. Chem., Int. Ed. 2025, e202420483; - PubMed
- h) Lal N., Deepshikha, Singh P., Shaikh A. C., Chem. Commun. 2025, 61, 3005. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

