New Pseudomonas infections drive Pf phage transmission in CF airways
- PMID: 40261708
- PMCID: PMC12220970
- DOI: 10.1172/jci.insight.188146
New Pseudomonas infections drive Pf phage transmission in CF airways
Abstract
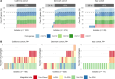
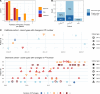
Pf bacteriophages, lysogenic viruses that infect Pseudomonas aeruginosa (Pa), are implicated in the pathogenesis of chronic Pa infections; phage-infected (Pf+) strains are known to predominate in people with cystic fibrosis (pwCF) who are older and have more severe disease. However, the transmission patterns of Pf underlying the progressive dominance of Pf+ strains are unclear. In particular, it is unknown whether phage transmission commonly occurs horizontally between bacteria via viral particles within the airway or whether Pf+ bacteria are mostly acquired via de novo Pseudomonas infections. Here, we studied Pa genomic sequences from 3 patient cohorts totaling 662 clinical isolates from 105 pwCF. We identified Pf+ isolates and analyzed transmission patterns of Pf within patients between genetically similar groups of bacteria called "clone types." We found that Pf was predominantly passed down vertically within Pa clone types and rarely via horizontal transfer between clone types within the airway. Conversely, we found extensive evidence of Pa de novo infection by a new, genetically distinct Pf+ Pa. Finally, we observed that clinical isolates showed reduced activity of type IV pili and reduced susceptibility to Pf in vitro. These results cast light on the transmission of virulence-associated phages in the clinical setting.
Keywords: Bacterial infections; Fibrosis; Infectious disease; Microbiology.
Figures



Similar articles
-
Pseudomonas superinfection drives Pf phage transmission within airway infections in patients with cystic fibrosis.bioRxiv [Preprint]. 2025 Jan 14:2025.01.14.632786. doi: 10.1101/2025.01.14.632786. bioRxiv. 2025. PMID: 39868244 Free PMC article. Preprint.
-
Prophages are infrequently associated with antibiotic resistance in Pseudomonas aeruginosa clinical isolates.mSphere. 2025 Mar 25;10(3):e0090424. doi: 10.1128/msphere.00904-24. Epub 2025 Feb 13. mSphere. 2025. PMID: 39945525 Free PMC article.
-
Pf bacteriophage is associated with decline in lung function in a longitudinal cohort of patients with cystic fibrosis and Pseudomonas airway infection.J Cyst Fibros. 2025 Mar;24(2):345-352. doi: 10.1016/j.jcf.2024.09.018. Epub 2024 Oct 25. J Cyst Fibros. 2025. PMID: 39490215 Free PMC article.
-
Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis.Cochrane Database Syst Rev. 2017 Apr 25;4(4):CD004197. doi: 10.1002/14651858.CD004197.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2023 Jun 2;6:CD004197. doi: 10.1002/14651858.CD004197.pub6. PMID: 28440853 Free PMC article. Updated.
-
Antibiotic treatment for non-tuberculous mycobacteria lung infection in people with cystic fibrosis.Cochrane Database Syst Rev. 2025 Mar 27;3(3):CD016039. doi: 10.1002/14651858.CD016039. Cochrane Database Syst Rev. 2025. PMID: 40145528
References
-
- Ackermann HW, DuBow MS. Viruses of Prokaryotes: General Properties of Bacteriophages. CRC Press; 1987.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

