Lymphatic dysfunction in lupus contributes to cutaneous photosensitivity and lymph node B cell responses
- PMID: 40261709
- PMCID: PMC12165800
- DOI: 10.1172/JCI168412
Lymphatic dysfunction in lupus contributes to cutaneous photosensitivity and lymph node B cell responses
Abstract
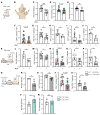
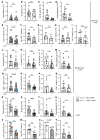
Patients with systemic lupus erythematosus (SLE) are photosensitive, developing skin inflammation with even ambient ultraviolet radiation (UVR), and this cutaneous photosensitivity can be associated with UVR-induced flares of systemic disease, which can involve increased autoantibodies and further end-organ injury. Mechanistic insight into the link between the skin responses and autoimmunity is limited. Signals from skin are transmitted directly to the immune system via lymphatic vessels, and here we show evidence for potentiation of UVR-induced lymphatic flow dysfunction in SLE patients and murine models. Improving lymphatic flow by manual lymphatic drainage (MLD) or with a transgenic model with increased lymphatic vessels reduces both cutaneous inflammation and lymph node B and T cell responses, and long-term MLD reduces splenomegaly and titers of a number of autoantibodies. Mechanistically, improved flow restrains B cell responses in part by stimulating a lymph node fibroblastic reticular cell-monocyte axis. Our results point to lymphatic modulation of lymph node stromal function as a link between photosensitive skin responses and autoimmunity and as a therapeutic target in lupus, provide insight into mechanisms by which the skin state regulates draining lymph node function, and suggest the possibility of MLD as an accessible and cost-effective adjunct to add to ongoing medical therapies for lupus and related diseases.
Keywords: Autoimmunity; Immunology; Inflammation; Lupus; Lymph; Vascular biology.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

