Promising New Anti-TIGIT Agents: Stealthy Allies in Cancer Immunotherapy
- PMID: 40261799
- PMCID: PMC12013639
- DOI: 10.1111/cts.70212
Promising New Anti-TIGIT Agents: Stealthy Allies in Cancer Immunotherapy
Abstract
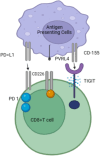
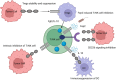
TIGIT (T cell immunoreceptor with immunoglobulin and tyrosine-based inhibitory motif (ITIM) domain), Vstm3, and VSIG9, are newly recognized immunological checkpoints. They are prominently expressed on CD4+ and CD8+ T cells, tumor-infiltrating lymphocytes (TILs), natural killer (NK) cells, and regulatory T cells (Tregs). The TIGIT (TIGIT) protein is crucial for immune modulation since it diminishes NK cell populations and hinders T cell activity in cancer patients and experimental models. CD155, the principal ligand of TIGIT in humans, has been recognized as a pivotal target for immunotherapy owing to its interaction with TIGIT. CD155 is linked to the efficacy of anti-programmed cell death protein 1 (PD-1) therapy, even without TIGIT expression, underscoring its importance in immune checkpoint suppression. Anti-TIGIT medicines, either independently or in conjunction with anti-PD-1 treatments, have demonstrated potential in augmenting immune responses to malignancies. This review examines the structural and functional characteristics of the TIGIT protein, new developments in anti-TIGIT drugs, and their prospective use in cancer immunotherapy.
Keywords: PD‐1; TIGIT; cancer; immunotherapy; resistance.
© 2025 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
T-cell immunoglobulin and ITIM domain as a target in combo anti-PD-(L)1 cancer therapy.Int J Biol Macromol. 2025 May;310(Pt 4):143557. doi: 10.1016/j.ijbiomac.2025.143557. Epub 2025 Apr 26. Int J Biol Macromol. 2025. PMID: 40294684 Review.
-
CD155-TIGIT Axis as a Therapeutic Target for Cancer Immunotherapy.Curr Med Chem. 2024;31(13):1634-1645. doi: 10.2174/0929867330666230324152532. Curr Med Chem. 2024. PMID: 38666504 Review.
-
Target therapy of TIGIT; a novel approach of immunotherapy for the treatment of colorectal cancer.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jan;398(1):231-241. doi: 10.1007/s00210-024-03346-7. Epub 2024 Aug 19. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39158733 Review.
-
Repositioning liothyronine for cancer immunotherapy by blocking the interaction of immune checkpoint TIGIT/PVR.Cell Commun Signal. 2020 Sep 7;18(1):142. doi: 10.1186/s12964-020-00638-2. Cell Commun Signal. 2020. PMID: 32894141 Free PMC article.
-
CD155/TIGIT, a novel immune checkpoint in human cancers (Review).Oncol Rep. 2021 Mar;45(3):835-845. doi: 10.3892/or.2021.7943. Epub 2021 Jan 19. Oncol Rep. 2021. PMID: 33469677 Review.
References
-
- Shaw G., Cavalcante L., Giles F. J., and Taylor A., “Elraglusib (9‐ING‐41), a Selective Small‐Molecule Inhibitor of Glycogen Synthase Kinase‐3 Beta, Reduces Expression of Immune Checkpoint Molecules PD‐1, TIGIT and LAG‐3 and Enhances CD8+ T Cell Cytolytic Killing of Melanoma Cells,” Journal of Hematology & Oncology 15, no. 1 (2022): 134, 10.1186/s13045-022-01352-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

