Computational Strategies for Broad Spectrum Venom Phospholipase A2 Inhibitors
- PMID: 40261809
- PMCID: PMC12076495
- DOI: 10.1021/acs.jcim.5c00045
Computational Strategies for Broad Spectrum Venom Phospholipase A2 Inhibitors
Abstract
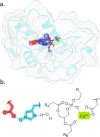
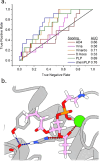
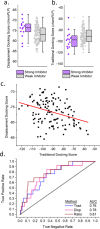
Snakebite envenoming is a persistent cause of mortality and morbidity worldwide due to the logistical challenges and costs of current antibody-based treatments. Their persistence motivates a broad interest in the discovery of inhibitors against multispecies venom phospholipase A2 (PLA2), which are underway as an alternative or supplemental treatment to improve health outcomes. Here, we present new computational strategies for improved inhibitor classification for challenging metalloenzyme targets across many species, including both a new method to utilize existing molecular docking, and subsequent data normalization. These methods were improved to support experimental screening efforts estimating the broader efficacy of candidate PLA2 inhibitors against diverse viper and elapid venoms.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Chippaux J.-P.; Massougbodji A.; Habib A. G.. The WHO Strategy for Prevention and Control of Snakebite Envenoming: A Sub-Saharan Africa Plan. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25. 10.1590/1678-9199-jvatitd-2019-0083. - DOI - PMC - PubMed
- Williams D. J.; Faiz M. A.; Abela-Ridder B.; Ainsworth S.; Bulfone T. C.; Nickerson A. D.; Habib A. G.; Junghanss T.; Fan H. W.; Turner M.; Harrison R. A.; Warrell D. A. Strategy for a Globally Coordinated Response to a Priority Neglected Tropical Disease: Snakebite Envenoming. PLoS Negl. Trop. Dis. 2019, 13, e0007059 10.1371/journal.pntd.0007059. - DOI - PMC - PubMed
-
- Wang J.-L. Antivenom Treatment for Snakebite Envenoming. Nat. Rev. Dis. Primers 2024, 10, 59. 10.1038/s41572-024-00543-9. - DOI - PubMed
- Hamza M.; Knudsen C.; Gnanathasan C. A.; Monteiro W.; Lewin M. R.; Laustsen A. H.; Habib A. G. Clinical Management of Snakebite Envenoming: Future Perspectives. Toxicon 2021, 11, 100079. 10.1016/j.toxcx.2021.100079. - DOI - PMC - PubMed
- Potet J.; Beran D.; Ray N.; Alcoba G.; Habib A. G.; Iliyasu G.; Waldmann B.; Ralph R.; Faiz M. A.; Monteiro W. M.; de Almeida Gonçalves Sachett J.; di Fabio J. L.; Cortés M. d. l. Á.; Brown N. I.; Williams D. J. Access to Antivenoms in the Developing World: A Multidisciplinary Analysis. Toxicon: X 2021, 12, 100086. 10.1016/j.toxcx.2021.100086. - DOI - PMC - PubMed
- Dalhat M. M.; Potet J.; Mohammed A.; Chotun N.; Tesfahunei H. A.; Habib A. G. Availability, Accessibility and Use of Antivenom for Snakebite Envenomation in Africa with Proposed Strategies to Overcome the Limitations. Toxicon: X 2023, 18, 100152. 10.1016/j.toxcx.2023.100152. - DOI - PMC - PubMed
- Habib A. G.; Musa B. M.; Iliyasu G.; Hamza M.; Kuznik A.; Chippaux J.-P. Challenges and Prospects of Snake Antivenom Supply in Sub-Saharan Africa. PLoS Negl. Trop. Dis. 2020, 14, e0008374 10.1371/journal.pntd.0008374. - DOI - PMC - PubMed
-
- Ryan R. Y. M.; Seymour J.; Loukas A.; Lopez J. A.; Ikonomopoulou M. P.; Miles J. J. Immunological Responses to Envenomation. Front. Immunol. 2021, 12, 661082. 10.3389/fimmu.2021.661082. - DOI - PMC - PubMed
- Knudsen C.; Laustsen A. Recent Advances in Next Generation Snakebite Antivenoms. Infect. Dis. Trop. Med. 2018, 3, 42. 10.3390/tropicalmed3020042. - DOI - PMC - PubMed
-
- Albulescu L.-O.; Westhorpe A.; Marriott A.; Clare R. H.; Stars E.; Mosallam N.; Chong-Jun-Weng D.; Gunasekar R.; Dawson C. A.; Woodley C.; James N.; Patel R.; Kool J.; Berry N. G.; O’Neill P. M.; Casewell N. R. Small Molecule Therapeutics for Neutralising Venom Toxins—A Drug Discovery Approach. Toxicon 2024, 248, 107942. 10.1016/j.toxicon.2024.107942. - DOI
- Xie C.; Albulescu L.-O.; Bittenbinder M. A.; Somsen G. W.; Vonk F. J.; Casewell N. R.; Kool J. Neutralizing Effects of Small Molecule Inhibitors and Metal Chelators on Coagulopathic Viperinae Snake Venom Toxins. Biomedicines 2020, 8, 297. 10.3390/biomedicines8090297. - DOI - PMC - PubMed
- Lewin M.; Samuel S.; Merkel J.; Bickler P. Varespladib (LY315920) Appears to be a Potent, Broad-Spectrum, Inhibitor of Snake Venom Phospholipase A2 and a Possible Pre-Referral Treatment for Envenomation. Toxins 2016, 8, 248. 10.3390/toxins8090248. - DOI - PMC - PubMed
- Hall S. R.; Rasmussen S. A.; Crittenden E.; Dawson C. A.; Bartlett K. E.; Westhorpe A. P.; Albulescu L.-O.; Kool J.; Gutiérrez J. M.; Casewell N. R. Repurposed Drugs and Their Combinations Prevent Morbidity-Inducing Dermonecrosis Caused by Diverse Cytotoxic Snake Venoms. Nat. Commun. 2023, 14, 7812. 10.1038/s41467-023-43510-w. - DOI - PMC - PubMed
-
- Isbister G. K.; Mirajkar N.; Fakes K.; Brown S. G. A.; Veerati P. C. Phospholipase A2 (PLA2) as an Early Indicator of Envenomation in Australian Elapid Snakebites (ASP-27). Biomedicines 2020, 8, 459. 10.3390/biomedicines8110459. - DOI - PMC - PubMed
- Castro-Amorim J.; Novo de Oliveira A.; Da Silva S. L.; Soares A. M.; Mukherjee A. K.; Ramos M. J.; Fernandes P. A. Catalytically Active Snake Venom PLA2 Enzymes: An Overview of Its Elusive Mechanisms of Reaction. J. Med. Chem. 2023, 66, 5364–5376. 10.1021/acs.jmedchem.3c00097. - DOI - PMC - PubMed
- Harris J.; Scott-Davey T. Secreted Phospholipases A2 of Snake Venoms: Effects on the Peripheral Neuromuscular System with Comments on the Role of Phospholipases A2 in Disorders of the CNS and Their Uses in Industry. Toxins 2013, 5, 2533–2571. 10.3390/toxins5122533. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

