Assessing therapeutic decisions in generalized myasthenia gravis: Study protocol
- PMID: 40261907
- PMCID: PMC12013924
- DOI: 10.1371/journal.pone.0322168
Assessing therapeutic decisions in generalized myasthenia gravis: Study protocol
Abstract
Background: The therapeutic landscape in generalized myasthenia gravis (gMG) has been continuously evolving in recent years, with over five products approved, each with different efficacy, safety, and administration profiles. With the availability of new targeted treatments, physicians are faced with the challenge of therapeutic decision-making tailored to traditional therapeutic goals, individual preferences, and personal experience, seeking optimal disease control with a positive safety profile. In this context of uncertainty and multiple novel choices, this study aims to provide insights into the preferred treatment choices of neurologists managing gMG and to identify demographic, professional or behavioral factors influencing the decision-making process.
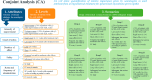
Methods: This is a non-interventional, cross-sectional, web-based study involving 150 neurologists treating gMG patients in collaboration with the Spanish Society of Neurology. The primary endpoint will be to assess preferences for different gMG treatment attributes using a conjoint analysis to create hypothetical treatment scenarios. Therapeutic inertia, described as the lack of treatment initiation or intensification when therapeutic goals are not met, will be evaluated as a secondary endpoint through 7 case scenarios simulating real gMG clinical practice situations. Neurologists will also answer a survey composed of demographic, professional, and behavioral characteristics (user resistance behavior, care-related regret, burnout, risk attitude, empathy, work fulfilment, and personality traits) to recognize possible factors influencing decisions.
Conclusions: The study findings will contribute to better understanding of decision-making in gMG under a changing therapeutic landscape with multiple new targeted options, and will identify which factors have a role in affecting those decisions.
Copyright: © 2025 Gutiérrez-Gutiérrez et al.. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: GGG has received compensation for consulting services from CSL Behring, Biogen, Alter, Takeda, Akcea, Lupin Neuroscience, Roche, Alexion, and Argenx; congresses support from Alter, Esteve, Sanofi-Genzyme, Pfizer, and UCB Pharma; has scientific relation with Lilly, Alexion, Genzyme, Takeda, Biogen, Pfizer, and Alter; books with Exeltis, Alter, Esteve, Andrómaco, and Bristol-Myers; and has received grants and awards from Lilly, UCB Pharma, and CSL Behring. RGB, ES, PDA, IE and JM are employees of Roche Pharma Spain. JS has received travel/congress support and compensation for consulting services from Roche, Biogen, UCB, and Argenx. AA has received speaking honoraria, consultation services compensation, or travel support for congress and scientific meetings attendance from Almirall, Bayer, Biogen, BMS, Janssen, Merck, Novartis, Roche, Sanofi, and Teva. MS and PB are employees of IQVIA Spain, the contract research organization selected by Roche for this study. LQ received speaker honoraria from Merck, Sanofi, Roche, Biogen, Grifols and CSL Behring; provided expert testimony for Grifols, Johnson & Johnson, Annexon Pharmaceuticals, Sanofi, Novartis, Takeda, and CSL-Behring; and received research funds from Roche, UCB, and Grifols. The rest of the authors declare no conflict of interest for this work. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- Dresser L, Wlodarski R, Rezania K, Soliven B. Myasthenia gravis: epidemiology, pathophysiology and clinical manifestations. J Clin Med. 2021;10(11):2235. https://doi.org/10.3390/jcm10112235 PMID: 34064035 - PMC - PubMed
-
- Narayanaswami P, Sanders D, Wolfe G, Benatar M, Cea G, Evoli A. International consensus guidance for management of myasthenia gravis: 2020 update. Neurology. 2021;96(3):114–22. https://doi.org/10.1212/WNL.0000000000011124 PMID: 33144515. - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

