The protein kinases KIPK and KIPK-LIKE1 suppress overbending during negative hypocotyl gravitropic growth in Arabidopsis
- PMID: 40261964
- PMCID: PMC12013712
- DOI: 10.1093/plcell/koaf056
The protein kinases KIPK and KIPK-LIKE1 suppress overbending during negative hypocotyl gravitropic growth in Arabidopsis
Abstract
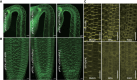
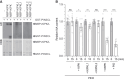
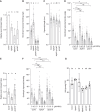
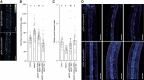
Plants use environmental cues to orient organ and plant growth, such as the direction of gravity or the direction, quantity, and quality of light. During the germination of Arabidopsis thaliana seeds in soil, negative gravitropism responses direct hypocotyl elongation such that the seedling can reach the light for photosynthesis and autotrophic growth. Similarly, hypocotyl elongation in the soil also requires mechanisms to efficiently grow around obstacles such as soil particles. Here, we identify KIPK (KINESIN-LIKE CALMODULIN-BINDING PROTEIN-INTERACTING PROTEIN KINASE) and the paralogous KIPKL1 (KIPK-LIKE1) as genetically redundant regulators of gravitropic hypocotyl bending. Moreover, we demonstrate that the homologous KIPKL2 (KIPK-LIKE2), which shows strong sequence similarity, must be functionally distinct. KIPK and KIPKL1 are polarly localized plasma membrane-associated proteins that can activate PIN-FORMED auxin transporters. KIPK and KIPKL1 are required to efficiently align hypocotyl growth with the gravity vector when seedling hypocotyls are grown on media plates or in soil, where contact with soil particles and obstacle avoidance impede direct negative gravitropic growth. Therefore, the polar KIPK and KIPKL1 kinases have different biological functions from the related AGC1 family kinases D6PK (D6 PROTEIN KINASE) or PAX (PROTEIN KINASE ASSOCIATED WITH BRX).
© The Author(s) 2025. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures



References
-
- Abas L, Benjamins R, Malenica N, Paciorek T, Wisniewska J, Moulinier-Anzola JC, Sieberer T, Friml J, Luschnig C. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol. 2006:8(3):249–256. 10.1038/ncb1369 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

