Fluorescence-barcoded cell lines stably expressing membrane-anchored influenza neuraminidases
- PMID: 40262372
- PMCID: PMC12086040
- DOI: 10.1016/j.vaccine.2025.127157
Fluorescence-barcoded cell lines stably expressing membrane-anchored influenza neuraminidases
Abstract
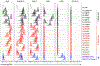
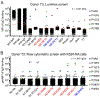
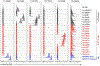
The discovery of broadly protective antibodies to the influenza virus neuraminidase (NA) has raised interest in NA as a vaccine target. However, recombinant, solubilized tetrameric NA ectodomains are often challenging to express and isolate, hindering the study of anti-NA humoral responses. To address this obstacle, we established a panel of 22 non-adherent cell lines stably expressing native, historical N1, N2, N3, N9, and NB NAs anchored on the cell surface. The cell lines are barcoded with fluorescent proteins, enabling high-throughput, 16-plex analyses of antibody binding with commonly available flow cytometers. The cell lines were at least as efficient as a Luminex multiplex binding assay at identifying NA antibodies from a library of unselected clonal IgGs derived from human memory B cells. The cell lines were also useful for measuring the magnitude and breadth of the serum antibody response elicited by experimental infection of rhesus macaques with influenza virus. The membrane-anchored NAs are catalytically active and are compatible with established sialidase activity assays. NA-expressing K530 cell lines therefore represent a useful tool for studying NA immunity and evaluating influenza vaccine efficacy.
Keywords: Barcode; Barcoding; Binding assays; ELLA; Flow cytometry; Fluorescent protein; Immunoassay; NA-Star assay; Sialidase; Vaccine.
Copyright © 2025 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Emmanuel B Walter reports a relationship with Pfizer that includes: consulting or advisory and funding grants. Emmanuel B Walter reports a relationship with Moderna Inc that includes: funding grants. Emmanuel B Walter reports a relationship with Seqirus that includes: funding grants. Emmanuel B Walter reports a relationship with Najit Technologies that includes: funding grants. Emmanuel B Walter reports a relationship with Clinetic that includes: funding grants. Emmanuel B Walter reports a relationship with Vaxcyte that includes: consulting or advisory. Emmanuel B Walter reports a relationship with ILiAD Biotechnologies that includes: consulting or advisory. Emmanuel B Walter reports a relationship with Shionogi that includes: consulting or advisory. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Update of
-
Fluorescence-barcoded cell lines stably expressing membrane-anchored influenza neuraminidases.bioRxiv [Preprint]. 2025 Apr 15:2025.01.01.631020. doi: 10.1101/2025.01.01.631020. bioRxiv. 2025. Update in: Vaccine. 2025 May 22;56:127157. doi: 10.1016/j.vaccine.2025.127157. PMID: 39803488 Free PMC article. Updated. Preprint.
References
-
- Caini S et al. , Probable extinction of influenza B/Yamagata and its public health implications: a systematic literature review and assessment of global surveillance databases. Lancet Microbe 5, 100851 (2024). - PubMed
-
- Monto AS, Zambon M, Weir JP, The End of B/Yamagata Influenza Transmission - Transitioning from Quadrivalent Vaccines. N Engl J Med 390, 1256–1258 (2024). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

