Human iPS cell-derived respiratory organoids as a model for respiratory syncytial virus infection
- PMID: 40262853
- PMCID: PMC12015132
- DOI: 10.26508/lsa.202402837
Human iPS cell-derived respiratory organoids as a model for respiratory syncytial virus infection
Abstract
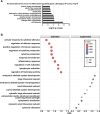
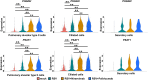
Respiratory syncytial virus (RSV) is a seasonal respiratory pathogen that primarily affects young children, potentially causing severe lower respiratory tract disease. Despite the high disease burden, understanding of RSV pathophysiology remains limited. To address this, advanced RSV infection models are needed. Whereas HEp-2 cells are widely used because of their high susceptibility to RSV, they do not accurately reflect the host response of the human respiratory tract. In this study, we evaluated human-induced pluripotent stem cell-derived respiratory organoids, which contain respiratory epithelial cells, immune cells, fibroblasts, and vascular endothelial cells, for their potential to model RSV infection and support pharmaceutical research. RSV-infected organoids exhibited high viral genome and protein expression, epithelial layer destruction, and increased collagen accumulation. Pro-inflammatory cytokine levels in culture supernatants also increased post-infection. Furthermore, RSV infection was significantly inhibited by monoclonal antibodies (nirsevimab, palivizumab, suptavumab, or clesrovimab), although ribavirin showed limited efficacy. These findings highlight the utility of respiratory organoids for RSV research.
© 2025 Hashimoto et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
-
- Alcorn JL, Stark JM, Chiappetta CL, Jenkins G, Colasurdo GN (2005) Effects of rsv infection on pulmonary surfactant protein sp-a in cultured human type ii cells: Contrasting consequences on sp-a mrna and protein. Am J Physiol Lung Cell Mol Physiol 289: L1113–L1122. 10.1152/ajplung.00436.2004 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
