Probing substrate water access through the O1 channel of Photosystem II by single site mutations and membrane inlet mass spectrometry
- PMID: 40263146
- PMCID: PMC12014804
- DOI: 10.1007/s11120-025-01147-4
Probing substrate water access through the O1 channel of Photosystem II by single site mutations and membrane inlet mass spectrometry
Abstract
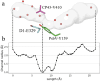
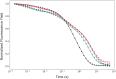
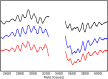
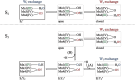
Light-driven water oxidation by photosystem II sustains life on Earth by providing the electrons and protons for the reduction of CO2 to carbohydrates and the molecular oxygen we breathe. The inorganic core of the oxygen evolving complex is made of the earth-abundant elements manganese, calcium and oxygen (Mn4CaO5 cluster), and is situated in a binding pocket that is connected to the aqueous surrounding via water-filled channels that allow water intake and proton egress. Recent serial crystallography and infrared spectroscopy studies performed with PSII isolated from Thermosynechococcus vestitus (T. vestitus) support that one of these channels, the O1 channel, facilitates water access to the Mn4CaO5 cluster during its S2→S3 and S3→S4→S0 state transitions, while a subsequent CryoEM study concluded that this channel is blocked in the cyanobacterium Synechocystis sp. PCC 6803, questioning the role of the O1 channel in water delivery. Employing site-directed mutagenesis we modified the two O1 channel bottleneck residues D1-E329 and CP43-V410 (T. vestitus numbering) and probed water access and substrate exchange via time resolved membrane inlet mass spectrometry. Our data demonstrates that water reaches the Mn4CaO5 cluster via the O1 channel in both wildtype and mutant PSII. In addition, the detailed analysis provides functional insight into the intricate protein-water-cofactor network near the Mn4CaO5 cluster that includes the pentameric, near planar 'water wheel' of the O1 channel.
Keywords: CP43-V410; D1-E329; O1 channel; Oxygen evolving complex; Photosystem II; Substrate water exchange; Synechocystis sp. PCC 6803; Water delivery; Water oxidation; Water wheel.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: Johannes Messinger is the editor-in-chief of Photosynthesis Research but not involved in evaluating this submission.
Figures



Similar articles
-
Network of hydrogen bonds near the oxygen-evolving Mn(4)CaO(5) cluster of photosystem II probed with FTIR difference spectroscopy.Biochemistry. 2014 Feb 18;53(6):1001-17. doi: 10.1021/bi401450y. Epub 2014 Feb 5. Biochemistry. 2014. PMID: 24460511
-
The Low Oxidation State Paradigm is More Consistent with XFEL Observations of the S₃ → [S₄] → S₀ Transition in Photosystem II.Chemistry. 2025 Jul 8;31(38):e202501010. doi: 10.1002/chem.202501010. Epub 2025 Jun 18. Chemistry. 2025. PMID: 40465289 Free PMC article.
-
Evidence from FTIR difference spectroscopy that D1-Asp61 influences the water reactions of the oxygen-evolving Mn4CaO5 cluster of photosystem II.Biochemistry. 2014 May 13;53(18):2941-55. doi: 10.1021/bi500309f. Epub 2014 Apr 23. Biochemistry. 2014. PMID: 24730551
-
Evolutionary diversity of proton and water channels on the oxidizing side of photosystem II and their relevance to function.Photosynth Res. 2023 Nov;158(2):91-107. doi: 10.1007/s11120-023-01018-w. Epub 2023 Jun 2. Photosynth Res. 2023. PMID: 37266800 Free PMC article. Review.
-
Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults.Cochrane Database Syst Rev. 2016 Apr 21;4(4):CD009016. doi: 10.1002/14651858.CD009016.pub2. Cochrane Database Syst Rev. 2016. PMID: 27098439 Free PMC article.
References
-
- Boussac A, Ugur I, Marion A et al (2018) The low spin - high spin equilibrium in the S2-state of the water oxidizing enzyme. Biochim Biophys Acta Bioenerg 1859:342–356. 10.1016/j.bbabio.2018.02.010 - PubMed

