Sex-specific formulations of doxazosin mesylate via direct powder extrusion 3D printing
- PMID: 40263229
- PMCID: PMC12350568
- DOI: 10.1007/s13346-025-01862-4
Sex-specific formulations of doxazosin mesylate via direct powder extrusion 3D printing
Abstract
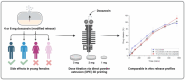
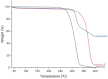
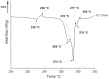
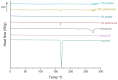
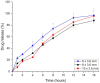
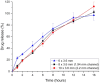
Males and females are known to exhibit significant differences in drug pharmacokinetics and pharmacodynamics, which are still overlooked in pharmaceutical research and development. These disparities contribute to adverse effects and increased mortality in females, highlighting the critical need for sex-specific formulations. Extended-release formulations of doxazosin mesylate, an alpha blocker used to treat hypertension, have shown significant sex-based differences in pharmacokinetics, leading to heightened adverse effects in females and rendering current titration recommendations impractical. This study explored the potential of a 3D printing (3DP) technology, direct powder extrusion (DPE), for producing personalised, sex-specific doses of doxazosin mesylate. A simple three component formulation was made composed of hydroxypropyl cellulose (HPC) polymer Klucel JF, D-mannitol, and doxazosin mesylate. Extended-release printlets of varying doses (1, 2, and 3 mg) were manufactured from a single 1% w/w doxazosin pharma-ink batch, enabling easy dose personalisation by adjusting the printlet dimensions. The use of a single pharma-ink supports the technology's ease of use in a pharmacy setting, by eliminating frequent pharma-ink changes during the pharmaceutical compounding process. In vitro dissolution testing revealed an extended drug release profile, influenced by surface-area-to-volume (SA: V) ratios. Introducing channels in larger printlets standardized the SA: V ratios, enhancing release profile uniformity. Release kinetics followed the Hixson-Crowell and Korsmeyer-Peppas models, indicating diffusion and polymer swelling mechanisms. This work highlights the capability of DPE 3DP for creating personalized, extended-release oral dosage forms, supporting precise dose customization for patient-specific therapy. Graphical Abstract.
Keywords: Additive manufacturing of oral drug products; Gender specific drug prescribing; Hypertension; Modified release formulations and pharmaceuticals; Personalized therapeutics; Three-dimensional printing of medications.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: No ethical approval was required for this study, and no consent to participation was needed. Consent for publication: No individuals participated in this work, therefore no consent for publication was needed. Competing interests: The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Alvaro Goyanes reports a relationship with FABRX that includes equity or stocks. Abdul Basit reports a relationship with FABRX that includes equity or stocks.
Figures


Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Inkjet printing of pharmaceutical tattoos for the direct deposition of oestradiol onto skin in turner syndrome.Eur J Pharm Biopharm. 2025 Sep;214:114798. doi: 10.1016/j.ejpb.2025.114798. Epub 2025 Jun 19. Eur J Pharm Biopharm. 2025. PMID: 40543629
-
3D printing of dose-flexible crystalline solid dispersion tablets suitable for preclinical and first-in-human studies.J Pharm Sci. 2025 Aug 7;114(10):103943. doi: 10.1016/j.xphs.2025.103943. Online ahead of print. J Pharm Sci. 2025. PMID: 40782895
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Rademaker M. Do women have more adverse drug reactions?? Am J Clin Dermatol. 2001;2(6):349–51. - PubMed
-
- Madla CM et al. Let’s talk about sex: differences in drug therapy in males and females. Adv Drug Deliv Rev, 2021: p. 113804. - PubMed
-
- Ochs HR, et al. Diazepam kinetics in relation to age and sex. Pharmacology. 1981;23(1):24–30. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

