The Sex Inclusive Research Framework to address sex bias in preclinical research proposals
- PMID: 40263253
- PMCID: PMC12015461
- DOI: 10.1038/s41467-025-58560-5
The Sex Inclusive Research Framework to address sex bias in preclinical research proposals
Abstract
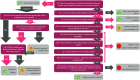
An interactive Sex Inclusive Research Framework (SIRF) supports the evaluation of in vivo and ex vivo research proposals to address the risk of sex bias in preclinical research. The framework delivers a traffic light classification, indicating whether a proposal is appropriate, risky, or insufficient with regard to sex inclusion. This tool is designed for use by researchers, (animal) ethical review boards, and funders to generate a rigorous and reproducible assessment of sex inclusion at the proposal level, thus helping address and resolve the embedded sex bias in preclinical research.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare the following competing interests: B.P., K.G. and N.A.K. have shareholdings in AstraZeneca. E.J.P. and N.P.d.S. are NC3Rs staff; whose role includes promoting the ARRIVE guidelines and the Experimental Design Assistant (EDA). N.A.K. and S.C.S. are members of the expert group who advise development of the EDA, S.C.S. is also the Chair of the EDA Project Management Board. The remaining authors declare no competing interests.
Figures
References
-
- Mogil, J. S. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat. Rev. Neurosci.21, 353–365 (2020). - PubMed
-
- Medical Research Council. Sex in experimental design—Guidance on new requirements. UK Research and Innovation https://www.ukri.org/councils/mrc/guidance-for-applicants/policies-and-g... (2022).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

