Injectable extracellular vesicle hydrogels with tunable viscoelasticity for depot vaccine
- PMID: 40263275
- PMCID: PMC12015221
- DOI: 10.1038/s41467-025-59278-0
Injectable extracellular vesicle hydrogels with tunable viscoelasticity for depot vaccine
Abstract
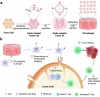
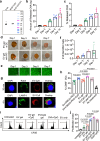
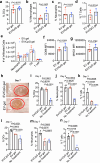
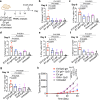
Extracellular vesicles (EVs) have been actively explored for therapeutic applications in the context of cancer and other diseases. However, the poor tissue retention of EVs has limited the development of EV-based therapies. Here we report a facile approach to fabricating injectable EV hydrogels with tunable viscoelasticity and gelation temperature, by metabolically tagging EVs with azido groups and further crosslinking them with dibenzocyclooctyne-bearing polyethylene glycol via efficient click chemistry. One such EV gel has a gelation temperature of 39.4 °C, enabling in situ gelation of solution-form EVs upon injection into the body. The in situ formed gels are stable for over 4 weeks and can attract immune cells including dendritic cells over time in vivo. We further show that tumor EV hydrogels, upon subcutaneous injection, can serve as a long-term depot for EV-encased tumor antigens, providing an extended time for the modulation of dendritic cells and subsequent priming of tumor-specific CD8+ T cells. The tumor EV hydrogel also shows synergy with anti-PD-1 checkpoint blockade for tumor treatment, and is able to reprogram the tumor microenvironment. As a proof-of-concept, we also demonstrate that EV hydrogels can induce enhanced antibody responses than solution-form EVs over an extended time. Our study yields a facile and universal approach to fabricating injectable EV hydrogels with tunable mechanics and improving the therapeutic efficacy of EV-based therapies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: H.W. and R.B. filed a patent application for the extracellular vesicle hydrogel technology. Other authors declare no competing interests.
Figures



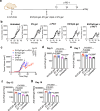
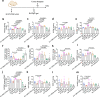
Similar articles
-
Injectable Supramolecular Ureidopyrimidinone Hydrogels Provide Sustained Release of Extracellular Vesicle Therapeutics.Adv Healthc Mater. 2019 Oct;8(20):e1900847. doi: 10.1002/adhm.201900847. Epub 2019 Sep 26. Adv Healthc Mater. 2019. PMID: 31559704
-
In situ modulation of dendritic cells by injectable thermosensitive hydrogels for cancer vaccines in mice.Biomacromolecules. 2014 Oct 13;15(10):3836-45. doi: 10.1021/bm501166j. Epub 2014 Sep 23. Biomacromolecules. 2014. PMID: 25207465 Free PMC article.
-
Metabolic tagging of extracellular vesicles and development of enhanced extracellular vesicle based cancer vaccines.Nat Commun. 2023 Dec 5;14(1):8047. doi: 10.1038/s41467-023-43914-8. Nat Commun. 2023. PMID: 38052869 Free PMC article.
-
Extracellular vesicle-loaded hydrogels for tissue repair and regeneration.Mater Today Bio. 2022 Dec 21;18:100522. doi: 10.1016/j.mtbio.2022.100522. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36593913 Free PMC article. Review.
-
Extracellular vesicles-based vaccines: Emerging immunotherapies against cancer.J Control Release. 2025 Feb 10;378:438-459. doi: 10.1016/j.jconrel.2024.12.010. Epub 2024 Dec 21. J Control Release. 2025. PMID: 39667569 Review.
Cited by
-
Lung cancer vaccine strategies: exploring the spectrum from traditional to RNA-based platforms.Front Bioeng Biotechnol. 2025 Jun 23;13:1617352. doi: 10.3389/fbioe.2025.1617352. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40625850 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- R21 CA270872/CA/NCI NIH HHS/United States
- R01CA274738/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R21CA270872/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 CA274738/CA/NCI NIH HHS/United States
- DMR 21-43673 CAR/National Science Foundation (NSF)
LinkOut - more resources
Full Text Sources
Research Materials

