Boosting hydrogel conductivity via water-dispersible conducting polymers for injectable bioelectronics
- PMID: 40263282
- PMCID: PMC12015517
- DOI: 10.1038/s41467-025-59045-1
Boosting hydrogel conductivity via water-dispersible conducting polymers for injectable bioelectronics
Erratum in
-
Author Correction: Boosting hydrogel conductivity via water-dispersible conducting polymers for injectable bioelectronics.Nat Commun. 2025 Jun 12;16(1):5308. doi: 10.1038/s41467-025-60718-0. Nat Commun. 2025. PMID: 40506425 Free PMC article. No abstract available.
Abstract
Bioelectronic devices hold transformative potential for healthcare diagnostics and therapeutics. Yet, traditional electronic implants often require invasive surgeries and are mechanically incompatible with biological tissues. Injectable hydrogel bioelectronics offer a minimally invasive alternative that interfaces with soft tissue seamlessly. A major challenge is the low conductivity of bioelectronic systems, stemming from poor dispersibility of conductive additives in hydrogel mixtures. We address this issue by engineering doping conditions with hydrophilic biomacromolecules, enhancing the dispersibility of conductive polymers in aqueous systems. This approach achieves a 5-fold increase in dispersibility and a 20-fold boost in conductivity compared to conventional methods. The resulting conductive polymers are molecularly and in vivo degradable, making them suitable for transient bioelectronics applications. These additives are compatible with various hydrogel systems, such as alginate, forming ionically cross-linkable conductive inks for 3D-printed wearable electronics toward high-performance physiological monitoring. Furthermore, integrating conductive fillers with gelatin-based bioadhesive hydrogels substantially enhances conductivity for injectable sealants, achieving 250% greater sensitivity in pH sensing for chronic wound monitoring. Our findings indicate that hydrophilic dopants effectively tailor conducting polymers for hydrogel fillers, enhancing their biodegradability and expanding applications in transient implantable biomonitoring.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

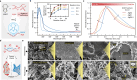



References
-
- Shi, J. et al. Monolithic-to-focal evolving biointerfaces in tissue regeneration and bioelectronics. Nat. Chem. Eng.1, 73–86 (2024). - DOI
-
- Li, P., Shi, J. & Tian, B. An electronic pill for non-invasive gastric monitoring. Nat. Electron.7, 434–435 (2024). - DOI
-
- Xu, S., Xiao, X., Manshaii, F. & Chen, J. Injectable fluorescent neural interfaces for cell-specific stimulating and imaging. Nano Lett.24, 4703–4716 (2024). - PubMed
MeSH terms
Substances
Grants and funding
- R01 HL149808/HL/NHLBI NIH HHS/United States
- R01 EB023052/EB/NIBIB NIH HHS/United States
- R01HL140618/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- T32EB023858/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 HL140618/HL/NHLBI NIH HHS/United States
- T32 EB027629/EB/NIBIB NIH HHS/United States
- R01 HL155815/HL/NHLBI NIH HHS/United States
- R01 EB031992/EB/NIBIB NIH HHS/United States
- R01 DC021461/DC/NIDCD NIH HHS/United States
- R01DC021461/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01EB023052/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01EB031992/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- T32 HL144449/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

