Shenfu injection alleviates lipopolysaccharide-induced liver injury in septic mice
- PMID: 40263344
- PMCID: PMC12015584
- DOI: 10.1038/s41598-025-98740-3
Shenfu injection alleviates lipopolysaccharide-induced liver injury in septic mice
Abstract
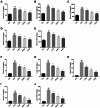
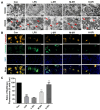
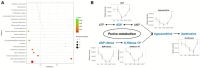
Shenfu injection (SFI) is a traditional Chinese medicine (TCM) for treating sepsis. The purpose of this study was to evaluate the protective effect of SFI on lipopolysaccharide (LPS)-induced liver injury in septic mice. The results showed that SFI intervention reduced liver/body weight and significantly improved the survival rate of septic mice. SFI could relieve the apoptosis of liver cells and ameliorate liver function in LPS-induced septic mice. SFI also diminished the serum and liver levels of the inflammatory factors IL-1β, IL-6, IL-18, IL-12, and TNF-α in a dose-dependent manner. SFI enhanced the mitochondrial membrane potential and alleviated the mitochondrial damage of liver in septic mice. Western blot revealed that the phosphorylation levels of IκB and NF-κB p65 increased significantly in the liver of LPS-induced septic mice. After SFI intervention, the phosphorylation levels of IκB and NF-κB p65 gradually recovered, especially at high concentration. SFI treatment reduced nuclear transduction, thus reducing transcriptional activity, which indicated that NF-κB p65 signal pathway might contribute to the anti-inflammatory and anti-apoptotic activities of SFI in the liver of LPS-induced septic mice. In addition, the metabolic profile of liver tissue in the model group was different from that in the control group, and SFI significantly regulated liver purine metabolism. These valuable findings suggested that SFI could improve mitochondrial function and mitigate inflammation and apoptosis, and thus alleviate LPS-induced liver injury in septic mice. SFI may be a promising drug to treat septic liver injury.
Keywords: Apoptosis; Inflammation; Liver injury; Purine metabolism; Sepsis; Shenfu injection; Traditional Chinese medicine.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: The study was conducted in accordance with ARRIVE guidelines and executed in compliance with the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University (IRB number: SYDW2019-229).
Figures





References
-
- Cecconi, M. et al. Sepsis and septic shock. Lancet392(10141), 75–87 (2018). - PubMed
-
- Luo, S. et al. Application of ultrasound-guided external jugular vein puncture in intensive care unit (ICU) patients with severe sepsis: A randomised trial. Ann. Palliat. Med.10(1), 530–537 (2021). - PubMed
-
- Xie, L. et al. Wenqingyin suppresses ferroptosis in the pathogenesis of sepsis-induced liver injury by activating the Nrf2-mediated signaling pathway. Phytomedicine114, 154748 (2023). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

