Three-dimensional bioprinted in vitro glioma tumor constructs for synchrotron microbeam radiotherapy dosimetry and biological study using gelatin methacryloyl hydrogel
- PMID: 40263410
- PMCID: PMC12015499
- DOI: 10.1038/s41598-025-88793-9
Three-dimensional bioprinted in vitro glioma tumor constructs for synchrotron microbeam radiotherapy dosimetry and biological study using gelatin methacryloyl hydrogel
Abstract
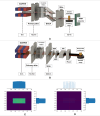
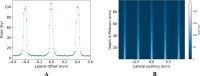
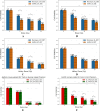
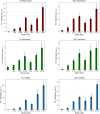
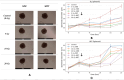
Synchrotron microbeam radiotherapy (MRT) is an innovative cancer treatment that uses micron-sized of ultra-high dose rate spatially fractionated X-rays to effectively control cancer growth while reducing the damage to surrounding healthy tissue. However, the current pre-clinical experiments are commonly limited with the use of conventional two-dimensional cell cultures which cannot accurately model in vivo tissue environment. This study aims to propose a three-dimensional (3D) bioprinting gelatin methacryloyl (GelMA) hydrogel protocol and to characterize 3D bioprinted glioma relative to cell monolayer and spheroid models for experimental MRT using 9L rat gliosarcoma and U87 human glioma. Synchrotron broad-beam (SBB) and MRT beams were delivered to all cell models using 5, 10, and 20 Gy. 3D bioprinting enables the creation of 3D cell models that mimic in vivo conditions using bioinks, biomaterials, and cells. Synchrotron dosimetry, Monte Carlo simulation, in vitro cell viability, and fluorescence microscopy were performed to understand the relationship of the radiation dosimetry with the radiobiological response of different cancer models. Encapsulated gliomas were placed inside 3D printed human and rat phantoms to mimic scattering conditions. Results showed that MRT kills more gliomas relative to SBB for all cell models. The 3D bioprinted culture detected the spatial clustering of dead cells due to MRT high peak doses as seen in fluorescence imaging. The result of this study progresses MRT research by integrating 3D bioprinting techniques in radiobiological experiments. The study's bioprinting protocol and results will help in reducing the use of animal experiments and possibly in clinical translation of MRT.
Keywords: 3D Printing; Biofabrication; Bioprinting; Brain Cancer; GelMA; Glioma; Microbeam Radiation Therapy; Spatial fractionation; Synchrotron Radiation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

